Abstract
Objective:
To investigate the anti-obesity effect of the adipokine zinc-α2-glycoprotein (ZAG) in rats and the mechanism of this effect.
Subjects:
Mature male Wistar rats (540±83 g) were administered human recombinant ZAG (50 μg per 100 g body weight given intravenously daily) for 10 days, while control animals received an equal volume of phosphate-buffered saline (PBS).
Results:
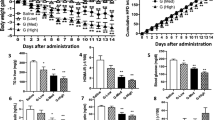
Animals treated with ZAG showed a progressive decrease in body weight, without a decrease in food and water intake, but with a 0.4 °C rise in body temperature. Body composition analysis showed loss of adipose tissue, but an increase in lean body mass. The loss of fat was due to an increase in lipolysis as shown by a 50% elevation of plasma glycerol, accompanied by increased utilization of non-esterified fatty acids, as evidenced by the 55% decrease in plasma levels. Plasma levels of glucose and triglycerides were also reduced by 36–37% and there was increased expression of the glucose transporter 4 in both skeletal muscle and adipose tissue. Expression of the lipolytic enzymes adipose triglyceride lipase and hormone-sensitive lipase in the white adipose tissue (WAT) were increased twofold after ZAG administration. There was almost a twofold increased expression of uncoupling proteins 1 and 3 in brown adipose tissue and WAT, which would contribute to increased substrate utilization. Administration of ZAG increased ZAG expression twofold in the gastrocnemius muscle, BAT and WAT, which was probably necessary for its biological effect.
Conclusion:
These results show that ZAG produces increased lipid mobilization and utilization in the rat.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
References
Rössner S . Obesity: the disease of the twenty-first century. Int J Obes 2002; 26 (Suppl 4): 52–54.
Hirai K, Hussey HJ, Barber MD, Price SA, Tisdale MJ . Biological evaluation of a lipid-mobilizing factor isolated from the urine of cancer patients. Cancer Res 1998; 58: 2359–2365.
Russell ST, Zimmerman TP, Domin BA, Tisdale MJ . Induction of lipolysis in vitro and loss of body fat in vivo by zinc-α2-glycoprotein. Biochim Biophys Acta 2004; 1636: 59–68.
Araki T, Gejyo F, Takagaki K, Haupt H, Schwick G, Burgi W et al. Complete amino acid sequence of human plasma zinc-α2-glycoprotein and its homology to histo-compatability antigens. Proc Natl Acad Sci USA 1988; 85: 679–683.
Todorov PT, McDevitt TM, Meyer DJ, Ueyama H, Ohkubo I, Tisdale MJ . Purification and characterization of a tumor lipid-mobilizing factor. Cancer Res 1998; 58: 2353–2358.
Bing C, Bao Y, Jenkins J, Sanders P, Manieri M, Cinti S et al. Zinc α2-glycoprotein, a lipid mobilizing factor, is expressed in adipocytes and is up-regulated in mice with cancer cachexia. Proc Natl Acad Sci USA 2004; 101: 2500–2505.
Russell ST, Tisdale MJ . The role of glucocorticoids in the induction of zinc-α2-glycoprotein expression in adipose tissue in cancer cachexia. Br J Cancer 2005; 92: 876–881.
Dahlman I, Kaaman M, Olsson T, Tan GD, Bickerton AST, Wahlen K et al. A unique role of moncyte chemoattractant protein 1 among chemokines in adipose tissue of obese subjects. J Endocrinol Metab 2005; 90: 5834–5840.
Mracek T, Ding Q, Tzanavari T, Kos K, Pinkney J, Wilding J et al. The adipokine zinc-α2-glycoprotein is downregulated with fat mass expansion in obesity. Clin Endocrinol 2010; 72: 334–341.
Gohda T, Makita Y, Shike T, Tanimoto M, Funabiki K, Horikoshi S et al. Identification of epistatic interaction involved in obesity using the KK/Ta mouse as a type 2 diabetes model. Is Zn-α2-glycoprotein-1 a candidate gene for obesity? Diabetes 2003; 52: 2175–2181.
Rolli V, Radosavljevic M, Astier V, Macquin C, Castan-Laurell I, Visentin V et al. Lipolysis is altered in MHC class I zinc-α2-glycoprotein deficient mice. FEBS Lett 2007; 581: 394–400.
Ueyama H, Naitoh H, Ohkubo I . Structure and expression of rat and mouse mRNAs for Zn-α2-glycoprotein. J Biochem 1994; 116: 677–681.
Beck SA, Tisdale MJ . Production of lipolytic and proteolytic factors by a murine tumor-producing cachexia in the host. Cancer Res 1987; 47: 5919–5923.
Wieland O . Glycerol UV method. In: Bergmeyer HU (ed.). Methods of Enzymatic Analysis. Academic Press: London, 1974. pp 1404–1409.
Russell ST, Tisdale MJ . Antidiabetic properties of zinc-α2-glycoprotein (ZAG) in ob/ob mice. Endocrinology 2010; 151: 948–957.
Lundholm K, Edström S, Karlberg I, Ekman L, Schersten T . Relationship of food intake, body composition and tumor growth to host metabolism in nongrowing mice with sarcoma. Cancer Res 1980; 40: 2515–2522.
Nisoli E, Tonello C, Landi M, Carruba MO . Functional studies of the first selective β3-adrenergic receptor antagonist SR59230A in rat brown adipocytes. Mol Pharmacol 1996; 44: 7–14.
Eley HL, Tisdale MJ . Skeletal muscle atrophy, a link between depression of protein synthesis and increase in degradation. J Biol Chem 2007; 282: 7087–7097.
Hassan MI, Waheed A, Yadav S, Singh TP, Ahmad F . Zinc α2-glcyoprotein: a multidisciplinary protein. Mol Cancer Res 2008; 6: 892–906.
Haemmerle G, Lass A, Zimmerman R, Gorkiewicz G, Meyer C, Rozman J et al. Defective lipolysis and altered energy metabolism in mice lacking adipose triglyceride lipase. Science 2006; 312: 734–737.
Osuga J, Ishibashi S, Oka T, Yagyu H, Tozawa R, Fujimoto A et al. Targeted disruption of hormone-sensitive lipase results in male sterility and adipocyte hypertrophy, but not in obesity. Proc Natl Acad Sci USA 2000; 97: 787–792.
Jocken JWE, Langin D, Smit E, Saris WHM, Valle C, Hul GB et al. Adipose triglyceride lipase and hormone-sensitive lipase protein expression is decreased in the obese insulin-resistant state. J Clin Endocinol Metab 2007; 92: 2292–2299.
Beck SA, Tisdale MJ . Effect of cancer cachexia on triacylglycerol/fatty acid substrate cycling in white adipose tissue. Lipids 2004; 39: 1187–1189.
Susulic VS, Frederich RC, Lawitts J, Tozze L, Kahn BB, Harper ME et al. Targeted disruption of the β3-adrenergic receptor gene. J Biol Chem 1995; 270: 29483–29492.
Marette A, Bukoqiecki LJ . Stimulation of glucose transport by insulin and norepinephrine in isolated rat brown adipocytes. Am J Physiol Cell Physiol 1989; 257: C714–C721.
Stephens L, Anderson K, Stokoe D, Erdjument-Bromage H, Painter GF, Holmes AB et al. Protein kinase B kinases that mediate phosphatidyl-inositol 3, 4, 5-triphosphate-dependent activation of protein kinase B. Science 1998; 279: 710–714.
Russell ST, Tisdale MJ . Effect of a tumour-derived lipid-mobilising factor on glucose and lipid metabolism in vivo. Br J Cancer 2002; 87: 580–584.
Russell ST, Hirai K, Tisdale MJ . Role of β3-adrenergic receptors in the action of a tumour lipid mobilising factor. Br J Cancer 2002; 86: 424–428.
Sanders PM, Tisdale MJ . Effect of zinc-α2-glycoprotein (ZAG) on expression of uncoupling proteins in skeletal muscle and adipose tissue. Cancer Lett 2004; 212: 71–81.
Ghorbani M, Himms-Hagen J . Appearance of brown adipocytes in white adipose tissue during CL316, 243-induced reversal of obesity and diabetes in Zucker fa/fa rats. Int J Obes 1997; 21: 465–475.
Inokuma K, Okamatsu-Ogura Y, Omachi A, Matsushita Y, Kimura K, Yamashita H et al. Indispensable role of mitochondrial UCP1 for antiobesity effect of β3-adrenrgic stimulation. Am J Physiol Endocrinol Metab 2006; 290: E1014–E1021.
Vallerand AL, Perusse F, Bukowiecki LJ . Cold exposure reverses inhibitory effects of fasting on peripheral glucose uptake in rats. Am J Physiol Regul Integr Comp Physiol 1989; 259: R1043–R1049.
Barbe P, Millet L, Galitzky J, Lafontan M, Berlan M . In situ assessment of the role of the β1-,β2-, and β3-adrenoreceptors in the control of lipolysis and nutritive blood flow in human subcutaneous adipose tissue. Br J Pharmacol 1996; 117: 907–913.
Weyer C, Tataranni PA, Snitker S, Danforth Jr E, Ravussin E . Increase in insulin action and fat oxidation after treatment with CL316, 243, a highly selective β3-adrenoreceptor agonist in humans. Diabetes 1998; 47: 1555–1561.
Revelli J-P, Muzzin P, Giacobino J-P . Modulation in vivo of β-adrenergic receptor subtypes in rat brown adipose tissue by the thermogenic agonist Ro16-8714. Biochem J 1992; 286: 743–7462.
Yeung DCY, Lam KSL, Wang Y, Tso AWK, Xu X . Serum zinc-alpha 2-glycoprotein correlates with adiposity, triglycerides and the key components of the metabolic syndrome in Chinese subjects. J Clin Endocrinol Metab 2009; 94: 2531–2536.
Russell ST, Tisdale MJ . The role of glucocorticoids in the induction of zinc-α2-glycoprotein expression in adipose tissue in cancer cachexia. Br J Cancer 2005; 92: 876–881.
Acknowledgements
We thank Mr Brian Burford for help with the animal experiments. This work was supported by a research grant from Halsa Pharmaceuticals, TX, USA.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Rights and permissions
About this article
Cite this article
Russell, S., Tisdale, M. Studies on the anti-obesity activity of zinc-α2-glycoprotein in the rat. Int J Obes 35, 658–665 (2011). https://doi.org/10.1038/ijo.2010.193
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ijo.2010.193
Keywords
This article is cited by
-
Associations of zinc-α-2-glycoprotein with metabolic syndrome and its components among adult Arabs
Scientific Reports (2022)
-
Correlation between plasma ZAG and adiponectin in older adults: gender modification and frailty specificity
BMC Geriatrics (2021)
-
Expression and Function of Zinc-α2-Glycoprotein
Neuroscience Bulletin (2019)
-
Zinc and diabetes mellitus: understanding molecular mechanisms and clinical implications
DARU Journal of Pharmaceutical Sciences (2015)
-
Zinc-alpha2-glycoprotein in patients with acute and chronic kidney disease
BMC Nephrology (2013)