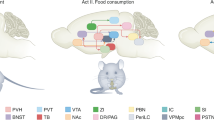
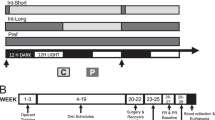
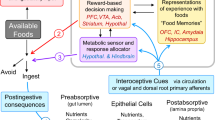
Abstract
Humans eat for many reasons, including the rewarding qualities of foods. A host of neurotransmitters have been shown to influence eating behavior and some of these appear to be involved in reward-induced eating. Endogenous opioid peptides and their receptors were first reported more than 30 years ago, and studies suggesting a role of opioids in the regulation of food intake date back nearly as far. Opioid agonists and antagonists have corresponding stimulatory and inhibitory effects on feeding. In addition to studies aimed at identifying the relevant receptor subtypes and sites of action within the brain, there has been a continuing interest in the role of opioids on diet/taste preferences, food reward, and the overlap of food reward with others types of reward. Data exist that suggest a role for opioids in the control of appetite for specific macronutrients, but there is also evidence for their role in the stimulation of intake based on already-existing diet or taste preferences and in controlling intake motivated by hedonics rather than by energy needs. Finally, various types of studies indicate an overlap between mechanisms mediating drug reward and palatable food reward. Preference or consumption of sweet substances often parallels the self-administration of several drugs of abuse, and under certain conditions, the termination of intermittent access to sweet substances produces symptoms that resemble those observed during opiate withdrawal. The overconsumption of readily available and highly palatable foods likely contributes to the growing rates of obesity worldwide. An understanding of the role of opioids in mediating food reward and promoting the overconsumption of palatable foods may provide insights into new approaches for preventing obesity.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout
Similar content being viewed by others
References
Marks-Kaufman R . Increased fat consumption induced by morphine administration in rats. Pharmacol Biochem Behav 1982; 16: 949–955.
Marks-Kaufman R, Kanarek RB . Modifications of nutrient selection induced by naloxone in rats. Psychopharmacology (Berl) 1981; 74: 321–324.
Gosnell BA, Krahn DD, Majchrzak MJ . The effects of morphine on diet selection are dependent upon baseline diet preferences. Pharmacol Biochem Behav 1990; 37: 207–212.
Glass MJ, Grace M, Cleary JP, Billington CJ, Levine AS . Potency of naloxone's anorectic effect in rats is dependent on diet preference. Am J Physiol 1996; 271: R217–R221.
Glass MJ, Billington CJ, Levine AS . Naltrexone administered to central nucleus of amygdala or PVN: neural dissociation of diet and energy. Am J Physiol Regul Integr Comp Physiol 2000; 279: R86–R92.
Naleid AM, Grace MK, Chimukangara M, Billington CJ, Levine AS . Paraventricular opioids alter intake of high-fat but not high-sucrose diet depending on diet preference in a binge model of feeding. Am J Physiol Regul Integr Comp Physiol 2007; 293: R99–105.
Woolley JD, Lee BS, Fields HL . Nucleus accumbens opioids regulate flavor-based preferences in food consumption. Neuroscience 2006; 143: 309–317.
Zhang M, Gosnell BA, Kelley AE . Intake of high-fat food is selectively enhanced by mu opioid receptor stimulation within the nucleus accumbens. J Pharmacol Exp Ther 1998; 285: 908–914.
Apfelbaum M, Mandenoff A . Naltrexone suppresses hyperphagia induced in the rat by a highly palatable diet. Pharmacol Biochem Behav 1981; 15: 89–91.
Levine AS, Weldon DT, Grace M, Cleary JP, Billington CJ . Naloxone blocks that portion of feeding driven by sweet taste in food-restricted rats. Am J Physiol 1995; 268: R248–R252.
Kirkham TC . Enhanced anorectic potency of naloxone in rats sham feeding 30% sucrose: reversal by repeated naloxone administration. Physiol Behav 1990; 47: 419–426.
Beczkowska IW, Koch JE, Bostock ME, Leibowitz SF, Bodnar RJ . Central opioid receptor subtype antagonists differentially reduce intake of saccharin and maltose dextrin solutions in rats. Brain Res 1993; 618: 261–270.
Gosnell BA, Majchrzak MJ . Centrally administered opioid peptides stimulate saccharin intake in nondeprived rats. Pharmacol Biochem Behav 1989; 33: 805–810.
Zhang M, Kelley AE . Opiate agonists microinjected into the nucleus accumbens enhance sucrose drinking in rats. Psychopharmacology (Berl) 1997; 132: 350–360.
Drewnowski A, Krahn DD, Demitrack MA, Nairn K, Gosnell BA . Taste responses and preferences for sweet high-fat foods: evidence for opioid involvement. Physiol Behav 1992; 51: 371–379.
Yeomans MR, Gray RW . Selective effects of naltrexone on food pleasantness and intake. Physiol Behav 1996; 60: 439–446.
Yeomans MR, Gray RW . Opioid peptides and the control of human ingestive behaviour. Neurosci Biobehav Rev 2002; 26: 713–728.
Kirkham TC, Blundell JE . Effect of naloxone and naltrexone on the development of satiation measured in the runway: comparisons with d-amphetamine and d-fenfluramine. Pharmacol Biochem Behav 1986; 25: 123–128.
Olszewski PK, Levine AS . Central opioids and consumption of sweet tastants: when reward outweighs homeostasis. Physiol Behav 2007; 91: 506–512.
Sabatier N . alpha-Melanocyte-stimulating hormone and oxytocin: a peptide signalling cascade in the hypothalamus. J Neuroendocrinol 2006; 18: 703–710.
Sclafani A, Rinaman L, Vollmer RR, Amico JA . Oxytocin knockout mice demonstrate enhanced intake of sweet and nonsweet carbohydrate solutions. Am J Physiol Regul Integr Comp Physiol 2007; 292: R1828–R1833.
Flanagan LM, Verbalis JG, Stricker EM . Naloxone potentiation of effects of cholecystokinin and lithium chloride on oxytocin secretion, gastric motility and feeding. Neuroendocrinology 1988; 48: 668–673.
Olszewski PK, Wirth MM, Grace MK, Levine AS, Giraudo SQ . Evidence of interactions between melanocortin and opioid systems in regulation of feeding. Neuroreport 2001; 12: 1727–1730.
Wardlaw SL, Kim J, Sobieszczyk S . Effect of morphine on proopiomelanocortin gene expression and peptide levels in the hypothalamus. Brain Res Mol Brain Res 1996; 41: 140–147.
Olszewski PK, Shi Q, Billington CJ, Levine AS . Opioids affect acquisition of LiCl-induced conditioned taste aversion: involvement of OT and VP systems. Am J Physiol Regul Integr Comp Physiol 2000; 279: R1504–R1511.
Civelli O . The orphanin FQ/nociceptin (OFQ/N) system. Results Probl Cell Differ 2008; 46: 1–25.
Pomonis JD, Billington CJ, Levine AS . Orphanin FQ, agonist of orphan opioid receptor ORL1, stimulates feeding in rats. Neuroreport 1996; 8: 369–371.
Colantuoni C, Schwenker J, McCarthy J, Rada P, Ladenheim B, Cadet JL et al. Excessive sugar intake alters binding to dopamine and mu-opioid receptors in the brain. Neuroreport 2001; 12: 3549–3552.
Wallace DL, Vialou V, Rios L, Carle-Florence TL, Chakravarty S, Kumar A et al. The influence of DeltaFosB in the nucleus accumbens on natural reward-related behavior. J Neurosci 2008; 28: 10272–10277.
Hajnal A, Smith GP, Norgren R . Oral sucrose stimulation increases accumbens dopamine in the rat. Am J Physiol Regul Integr Comp Physiol 2004; 286: R31–R37.
Lenoir M, Serre F, Cantin L, Ahmed SH . Intense sweetness surpasses cocaine reward. PLoS ONE 2007; 2: e698.
Jewett DC, Grace MK, Levine AS . Chronic sucrose ingestion enhances mu-opioid discriminative stimulus effects. Brain Res 2005; 1050: 48–52.
Avena NM, Hoebel BG . A diet promoting sugar dependency causes behavioral cross-sensitization to a low dose of amphetamine. Neuroscience 2003; 122: 17–20.
Gosnell BA . Sucrose intake enhances behavioral sensitization produced by cocaine. Brain Res 2005; 1031: 194–201.
Foley KA, Fudge MA, Kavaliers M, Ossenkopp KP . Quinpirole-induced behavioral sensitization is enhanced by prior scheduled exposure to sucrose: a multi-variable examination of locomotor activity. Behav Brain Res 2006; 167: 49–56.
DeSousa NJ, Bush DE, Vaccarino FJ . Self-administration of intravenous amphetamine is predicted by individual differences in sucrose feeding in rats. Psychopharmacology (Berl) 2000; 148: 52–58.
Gosnell BA . Sucrose intake predicts rate of acquisition of cocaine self-administration. Psychopharmacology (Berl) 2000; 149: 286–292.
Gosnell BA, Lane KE, Bell SM, Krahn DD . Intravenous morphine self-administration by rats with low versus high saccharin preferences. Psychopharmacology (Berl) 1995; 117: 248–252.
Carroll ME, Morgan AD, Lynch WJ, Campbell UC, Dess NK . Intravenous cocaine and heroin self-administration in rats selectively bred for differential saccharin intake: phenotype and sex differences. Psychopharmacology (Berl) 2002; 161: 304–313.
Nolan LJ, Scagnelli LM . Preference for sweet foods and higher body mass index in patients being treated in long-term methadone maintenance. Subst Use Misuse 2007; 42: 1555–1566.
Kampov-Polevoy AB, Garbutt JC, Janowsky DS . Association between preference for sweets and excessive alcohol intake: a review of animal and human studies. Alcohol Alcohol 1999; 34: 386–395.
Janowsky DS, Pucilowski O, Buyinza M . Preference for higher sucrose concentrations in cocaine abusing-dependent patients. J Psychiatr Res 2003; 37: 35–41.
Avena NM, Rada P, Hoebel BG . Evidence for sugar addiction: behavioral and neurochemical effects of intermittent, excessive sugar intake. Neurosci Biobehav Rev 2008; 32: 20–39.
Colantuoni C, Rada P, McCarthy J, Patten C, Avena NM, Chadeayne A et al. Evidence that intermittent, excessive sugar intake causes endogenous opioid dependence. Obes Res 2002; 10: 478–488.
Drewnowski A, Bellisle F . Is sweetness addictive? Nutr Bull 2007; 32: 52.
Popkin BM, Nielsen SJ . The sweetening of the world's diet. Obes Res 2003; 11: 1325–1332.
Kelly T, Yang W, Chen CS, Reynolds K, He J . Global burden of obesity in 2005 and projections to 2030. Int J Obes (Lond) 2008; 32: 1431–1437.
Acknowledgements
The writing of this paper was supported in part by the National Institute of Drug Abuse (R01DA021280), and the National Institute of Diabetes and Ingestive and Kidney Diseases (P30DK50456).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Gosnell, B., Levine, A. Reward systems and food intake: role of opioids. Int J Obes 33 (Suppl 2), S54–S58 (2009). https://doi.org/10.1038/ijo.2009.73
Published:
Issue Date:
DOI: https://doi.org/10.1038/ijo.2009.73
Keywords
This article is cited by
-
Emotion, motivation, decision-making, the orbitofrontal cortex, anterior cingulate cortex, and the amygdala
Brain Structure and Function (2023)
-
Obesity risk is associated with altered cerebral glucose metabolism and decreased μ-opioid and CB1 receptor availability
International Journal of Obesity (2022)
-
Cerebral μ-opioid and CB1 receptor systems have distinct roles in human feeding behavior
Translational Psychiatry (2021)
-
The Role of Positron Emission Tomography in Bariatric Surgery Research: a Review
Obesity Surgery (2021)
-
Pain influences food preference and food-related memory by activating the basolateral amygdala in rats
Experimental Brain Research (2021)