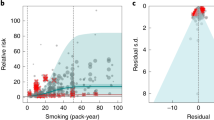
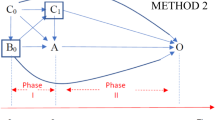
Abstract
Many observational studies have estimated a strong effect of obesity on mortality. In this paper, we explicitly define the causal question that is asked by these studies and discuss the problems associated with it. We argue that observational studies of obesity and mortality violate the condition of consistency of counterfactual (potential) outcomes, a necessary condition for meaningful causal inference, because (1) they do not explicitly specify the interventions on body mass index (BMI) that are being compared and (2) different methods to modify BMI may lead to different counterfactual mortality outcomes, even if they lead to the same BMI value in a given person. Besides precluding the estimation of unambiguous causal effects, this violation of consistency affects the ability to address two additional conditions that are also necessary for causal inference: exchangeability and positivity. We conclude that consistency violations not only preclude the estimation of well-defined causal effects but also compromise our ability to estimate ill-defined causal effects.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout
Similar content being viewed by others
References
Allison DB, Fontaine KR, Manson JE, Stevens J, VanItallie TB . Annual deaths attributable to obesity in the United States. J Am Med Assoc 1999; 282: 1530–1538.
Mokdad AH, Marks JS, Stroup DF, Gerberding JL . Actual causes of death in the United States, 2000. J Am Med Assoc 2004; 291: 1238–1245.
Mokdad AH, Marks JS, Stroup DF, Gerberding JL . Correction: actual causes of death in the United States, 2000. J Am Med Assoc 2005; 293: 293–294.
Flegal KM, Graubard BI, Williamson DF, Gail MH . Excess deaths associated with underweight, overweight, and obesity. J Am Med Assoc 2005; 293: 1861–1867.
Hernán MA . A definition of causal effect for epidemiological research. J Epidemiol Commun Health 2004; 58: 265–271.
Hernán MA, Robins JM . Estimating causal effects from epidemiological data. J Epidemiol Commun Health 2006; 60: 578–586.
Robins JM, Greenland S . Comment on ‘Causal inference without counterfactuals’ by AP Dawid. J Am Stat Assoc 2000; 95: 431–435.
Hernán MA . Invited commentary: hypothetical interventions to define causal effects: afterthought or prerequisite? Am J Epidemiol 2005; 162: 618–620.
Greenland S, Rothman KJ . Measures of effect and measures of association. In: Rothman KJ, Greenland S (eds). Modern Epidemiology, 2nd edn. Lippincott-Raven: Philadelphia, 1998, pp 47–64.
Greenland S . Epidemiologic measures and policy formulation: lessons from potential outcomes (with discussion). Emerging Themes Epidemiol 2005; 2: 5.
Holland PW . Statistics and causal inference. J Am Stat Assoc 1986; 81: 945–961.
Robins JM, Hernán MA . Estimation of the causal effects of time-varying exposures. In: Fitzmaurice G, Davidian M, Verbeke G, Molenberghs G (eds). Advances in Longitudinal Data Analysis. Chapman & Hall/CRC Press: New York, 2008 (in press).
Acknowledgements
We thank Sander Greenland, Sonia Hernández-Díaz and Karen Steinberg for their detailed comments and expert advice. This work was supported by NIH Grant R01 HL080644.
Author information
Authors and Affiliations
Corresponding author
Appendix
Appendix
Formal definitions
We say that a counterfactual outcome Ya is consistent with the actual or realized outcome Y if Ya=Y when the subject received exposure level A=a.
Exchangeability means that any counterfactual outcome under any treatment level a is independent of the treatment actually received A, which is written symbolically as Ya A. Randomization of the treatment is expected to result in exchangeability. Stratified randomization, in which the probability of receiving treatment varies by levels of the variables in L, is expected to result in conditional exchangeability within levels L, which is written as Ya
A. Randomization of the treatment is expected to result in exchangeability. Stratified randomization, in which the probability of receiving treatment varies by levels of the variables in L, is expected to result in conditional exchangeability within levels L, which is written as Ya A∣L.
A∣L.
For discrete treatment A and covariates L, the positivity condition is written as Pr[A=a∣L=l]>0 if Pr[L=l]≠0. In general, positivity is written as fA∣L(a∣l)>0 if fL(l)≠0, where fX∣Z(x∣z) is the conditional density function of the random variable X evaluated at the value x given the random variable Z evaluated at the value z.
Rights and permissions
About this article
Cite this article
Hernán, M., Taubman, S. Does obesity shorten life? The importance of well-defined interventions to answer causal questions. Int J Obes 32 (Suppl 3), S8–S14 (2008). https://doi.org/10.1038/ijo.2008.82
Published:
Issue Date:
DOI: https://doi.org/10.1038/ijo.2008.82
Keywords
This article is cited by
-
On the estimation of the effect of weight change on a health outcome using observational data, by utilising the target trial emulation framework
International Journal of Obesity (2023)
-
Body weight, weight change and the risk of cardiovascular disease in patients with hypertension: a primary-care cohort study
International Journal of Obesity (2023)
-
Causal Pluralism in Medicine and its Implications for Clinical Practice
Journal for General Philosophy of Science (2023)
-
Suicide Following the COVID-19 Pandemic Outbreak: Variation Across Place, Over Time, and Across Sociodemographic Groups. A Systematic Integrative Review
Current Psychiatry Reports (2023)
-
Estimating Causal Effects of Interventions on Early-life Environmental Exposures Using Observational Data
Current Environmental Health Reports (2022)