Abstract
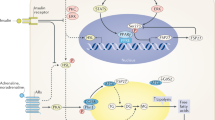
This review will focus on the recent findings in adipose tissue metabolism with special attention to human adipocyte biology and physiology. There are major advances stemming from the concomitant results obtained from studies on mature human adipocytes, human preadipocytes differentiated in vitro and murine adipose cell lines. Physiological developments have been based on the expanded utilization of various kinds of murine transgenic models and physiological techniques such as microdialysis, open-flow microperfusion, arteriovenous techniques and the utilization of deuterium- or tritium-labelled metabolites that have provided a number of physiological advances in the understanding of human adipose tissue physiology. Gene expression profiling studies and nutrigenomics are emerging methods that herald interesting approaches for the future. An overview of recent discoveries in the mechanisms involved in the control of free fatty acid uptake, triacylglycerol synthesis and fat deposition will be discussed, as well as recent advances in the mechanisms involved in the lipolytic pathways, the role of lipases and perilipins. In addition, the in vivo validation of catecholamine action and the discovery of the lipolytic effects of natriuretic peptides will also be covered.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
Rodbell M . Metabolism of isolated fat cells. I. Effects of hormones on glucose metabolism and lipolysis. J Biol Chem 1964; 239: 375–380.
Hauner H, Entenmann G, Wabitsch M, Gaillard D, Ailhaud G, Negrel R et al. Promoting effect of glucocorticoids on the differentiation of human adipocyte precursor cells cultured in a chemically defined medium. J Clin Invest 1989; 84: 1663–1670.
Sengenes C, Lolmede K, Zakaroff-Girard A, Busse R, Bouloumie A . Preadipocytes in the human subcutaneous adipose tissue display distinct features from the adult mesenchymal and hematopoietic stem cells. J Cell Physiol 2005; 205: 114–122.
Lonnroth P, Jansson PA, Smith U . A microdialysis method allowing characterization of intercellular water space in humans. Am J Physiol 1987; 253: E228–E231.
Arner P, Bolinder J, Eliasson A, Lundin A, Ungerstedt U . Microdialysis of adipose tissue and blood for in vivo lipolysis studies. Am J Physiol 1988; 255: E737–E742.
Bolinder J, Ungerstedt U, Arner P . Microdialysis measurement of the absolute glucose concentration in subcutaneous adipose tissue allowing glucose monitoring in diabetic patients. Diabetologia 1992; 35: 1177–1180.
Galitzky J, Lafontan M, Nordenstrom J, Arner P . Role of vascular alpha-2 adrenoceptors in regulating lipid mobilization from human adipose tissue. J Clin Invest 1993; 91: 1997–2003.
Barbe P, Millet L, Galitzky J, Lafontan M, Berlan M . In situ assessment of the role of the beta 1-, beta 2- and beta 3-adrenoceptors in the control of lipolysis and nutritive blood flow in human subcutaneous adipose tissue. Br J Pharmacol 1996; 117: 907–913.
Lafontan M, Arner P . Application of in situ microdialysis to measure metabolic and vascular responses in adipose tissue. Trends Pharmacol Sci 1996; 17: 309–313.
Schaupp L, Ellmerer M, Brunner GA, Wutte A, Sendlhofer G, Trajanoski Z et al. Direct access to interstitial fluid in adipose tissue in humans by use of open-flow microperfusion. Am J Physiol 1999; 276: E401–E408.
Ellmerer M, Schaupp L, Brunner GA, Sendlhofer G, Wutte A, Wach P et al. Measurement of interstitial albumin in human skeletal muscle and adipose tissue by open-flow microperfusion. Am J Physiol 2000; 278: E352–E356.
Coppack SW, Frayn KN, Humphreys SM, Dhar H, Hockaday TD . Effects of insulin on human adipose tissue metabolism in vivo. Clin Sci (London) 1989; 77: 663–670.
Frayn KN, Coppack SW, Humphreys SM, Whyte PL . Metabolic characteristics of human adipose tissue in vivo. Clin Sci (London) 1989; 76: 509–516.
Frayn KN, Coppack SW, Humphreys SM . Subcutaneous adipose tissue metabolism studied by local cartheterization. Int J Obesity 1993; 17 (Suppl 3): S18–S21.
Mittendorfer B, Liem O, Patterson BW, Miles JM, Klein S . What does the measurement of whole-body fatty acid rate of appearance in plasma by using a fatty acid tracer really mean? Diabetes 2003; 52: 1641–1648.
Strawford A, Antelo F, Christiansen M, Hellerstein MK . Adipose tissue triglyceride turnover, de novo lipogenesis, and cell proliferation in humans measured with 2H2O. Am J Physiol 2004; 286: E577–E588.
Votruba SB, Jensen MD . Regional fat deposition as a factor in FFA metabolism. Annu Rev Nutr 2007; 27: 149–163.
Mutch D . Identifying regulatory hubs in obesity with nutrigenomics. Curr Opin Endocrinol Diabetes 2006; 13: 431–437.
Mutch DM, Temanni MR, Henegar C, Combes F, Pelloux V, Holst C et al. Adipose gene expression prior to weight loss can differentiate and weakly predict dietary responders. PLoS ONE 2007; 2: e1344.
Sjostrom L . Fatty acid synthesis de novo in adipose tissue from obese subjects on a hypercaloric high-carbohydrate diet. Scand J Clin Lab Invest 1973; 32: 339–349.
Bjorntorp P, Sjostrom L . Carbohydrate storage in man: speculations and some quantitative considerations. Metabolism 1978; 27: 1853–1865.
Swierczynski J, Goyke E, Wach L, Pankiewicz A, Kochan Z, Adamonis W et al. Comparative study of the lipogenic potential of human and rat adipose tissue. Metabolism 2000; 49: 594–599.
Letexier D, Pinteur C, Large V, Frering V, Beylot M . Comparison of the expression and activity of the lipogenic pathway in human and rat adipose tissue. J Lipid Res 2003; 44: 2127–2134.
Wong H, Yang D, Hill JS, Davis RC, Nikazy J, Schotz MC . A molecular biology-based approach to resolve the subunit orientation of lipoprotein lipase. Proc Natl Acad Sci USA 1997; 94: 5594–5598.
Wong H, Schotz MC . The lipase gene family. J Lipid Res 2002; 43: 993–999.
Goldberg IJ . Lipoprotein lipase and lipolysis: central roles in lipoprotein metabolism and atherogenesis. J Lipid Res 1996; 37: 693–707.
Fielding BA, Frayn KN . Lipoprotein lipase and the disposition of dietary fatty acids. Br J Nutr 1998; 80: 495–502.
Pennacchio LA, Olivier M, Hubacek JA, Cohen JC, Cox DR, Fruchart JC et al. An apolipoprotein influencing triglycerides in humans and mice revealed by comparative sequencing. Science 2001; 294: 169–173.
Grosskopf I, Baroukh N, Lee SJ, Kamari Y, Harats D, Rubin EM et al. Apolipoprotein A-V deficiency results in marked hypertriglyceridemia attributable to decreased lipolysis of triglyceride-rich lipoproteins and removal of their remnants. Arterioscler Thromb Vasc Biol 2005; 25: 2573–2579.
Pennacchio LA, Olivier M, Hubacek JA, Krauss RM, Rubin EM, Cohen JC . Two independent apolipoprotein A5 haplotypes influence human plasma triglyceride levels. Hum Mol Genet 2002; 11: 3031–3038.
Marcais C, Verges B, Charriere S, Pruneta V, Merlin M, Billon S et al. Apoa5 Q139X truncation predisposes to late-onset hyperchylomicronemia due to lipoprotein lipase impairment. J Clin Invest 2005; 115: 2862–2869.
Merkel M, Heeren J . Give me A5 for lipoprotein hydrolysis! J Clin Invest 2005; 115: 2694–2696.
Merkel M, Loeffler B, Kluger M, Fabig N, Geppert G, Pennacchio LA et al. Apolipoprotein AV accelerates plasma hydrolysis of triglyceride-rich lipoproteins by interaction with proteoglycan-bound lipoprotein lipase. J Biol Chem 2005; 280: 21553–21560.
Ioka RX, Kang MJ, Kamiyama S, Kim DH, Magoori K, Kamataki A et al. Expression cloning and characterization of a novel glycosylphosphatidylinositol-anchored high density lipoprotein-binding protein, GPI-HBP1. J Biol Chem 2003; 278: 7344–7349.
Beigneux AP, Davies BS, Gin P, Weinstein MM, Farber E, Qiao X et al. Glycosylphosphatidylinositol-anchored high-density lipoprotein-binding protein 1 plays a critical role in the lipolytic processing of chylomicrons. Cell Metab 2007; 5: 279–291.
Reina M, Brunzell JD, Deeb SS . Molecular basis of familial chylomicronemia: mutations in the lipoprotein lipase and apolipoprotein C-II genes. J Lipid Res 1992; 33: 1823–1832.
Cunningham O, Andolfo A, Santovito ML, Iuzzolino L, Blasi F, Sidenius N . Dimerization controls the lipid raft partitioning of uPAR/CD87 and regulates its biological functions. EMBO J 2003; 22: 5994–6003.
Young SG, Davies BS, Fong LG, Gin P, Weinstein MM, Bensadoun A et al. GPIHBP1: an endothelial cell molecule important for the lipolytic processing of chylomicrons. Curr Opin Lipidol 2007; 18: 389–396.
Mead JR, Irvine SA, Ramji DP . Lipoprotein lipase: structure, function, regulation, and role in disease. J Mol Med 2002; 80: 753–769.
Sukonina V, Lookene A, Olivecrona T, Olivecrona G . Angiopoietin-like protein 4 converts lipoprotein lipase to inactive monomers and modulates lipase activity in adipose tissue. Proc Natl Acad Sci USA 2006; 103: 17450–17455.
Coppack SW, Evans RD, Fisher RM, Frayn KN, Gibbons GF, Humphreys SM et al. Adipose tissue metabolism in obesity: lipase action in vivo before and after a mixed meal. Metabolism 1992; 41: 264–272.
Ong JM, Kern PA . Effect of feeding and obesity on lipoprotein lipase activity, immunoreactive protein, and messenger RNA levels in human adipose tissue. J Clin Invest 1989; 84: 305–311.
Frayn KN . Adipose tissue as a buffer for daily lipid flux. Diabetologia 2002; 45: 1201–1210.
Kampf JP, Kleinfeld AM . Is membrane transport of FFA mediated by lipid, protein, or both? An unknown protein mediates free fatty acid transport across the adipocyte plasma membrane. Physiology (Bethesda, MD) 2007; 22: 7–14.
Civelek VN, Hamilton JA, Tornheim K, Kelly KL, Corkey BE . Intracellular pH in adipocytes: effects of free fatty acid diffusion across the plasma membrane, lipolytic agonists, and insulin. Proc Natl Acad Sci USA 1996; 93: 10139–10144.
Schaffer JE . Fatty acid transport: the roads taken. Am J Physiol 2002; 282: E239–E246.
Kleinfeld AM, Chu P, Romero C . Transport of long-chain native fatty acids across lipid bilayer membranes indicates that transbilayer flip-flop is rate limiting. Biochemistry 1997; 36: 14146–14158.
Hamilton JA, Guo W, Kamp F . Mechanism of cellular uptake of long-chain fatty acids: Do we need cellular proteins? Mol Cell Biochem 2002; 239: 17–23.
Kamp F, Hamilton JA . How fatty acids of different chain length enter and leave cells by free diffusion. Prostaglandins Leukot Essent Fatty Acids 2006; 75: 149–159.
Pohl J, Ring A, Hermann T, Stremmel W . Role of FATP in parenchymal cell fatty acid uptake. Biochim Biophys Acta 2004; 1686: 1–6.
Stump DD, Zhou SL, Berk PD . Comparison of plasma membrane FABP and mitochondrial isoform of aspartate aminotransferase from rat liver. Am J Physiol 1993; 265: G894–G902.
Stahl A, Evans JG, Pattel S, Hirsch D, Lodish HF . Insulin causes fatty acid transport protein translocation and enhanced fatty acid uptake in adipocytes. Dev Cell 2002; 2: 477–488.
Lewis SE, Listenberger LL, Ory DS, Schaffer JE . Membrane topology of the murine fatty acid transport protein 1. J Biol Chem 2001; 276: 37042–37050.
Coe NR, Smith AJ, Frohnert BI, Watkins PA, Bernlohr DA . The fatty acid transport protein (FATP1) is a very long chain acyl-CoA synthetase. J Biol Chem 1999; 274: 36300–36304.
Richards MR, Harp JD, Ory DS, Schaffer JE . Fatty acid transport protein 1 and long-chain acyl coenzyme A synthetase 1 interact in adipocytes. J Lipid Res 2006; 47: 665–672.
Lobo S, Wiczer BM, Smith AJ, Hall AM, Bernlohr DA . Fatty acid metabolism in adipocytes: functional analysis of fatty acid transport proteins 1 and 4. J Lipid Res 2007; 48: 609–620.
Martin G, Nemoto M, Gelman L, Geffroy S, Najib J, Fruchart JC et al. The human fatty acid transport protein-1 (SLC27A1; FATP-1) cDNA and gene: organization, chromosomal localization, and expression. Genomics 2000; 66: 296–304.
Martin G, Schoonjans K, Lefebvre AM, Staels B, Auwerx J . Coordinate regulation of the expression of the fatty acid transport protein and acyl-CoA synthetase genes by PPARalpha and PPARgamma activators. J Biol Chem 1997; 272: 28210–28217.
Man MZ, Hui TY, Schaffer JE, Lodish HF, Bernlohr DA . Regulation of the murine adipocyte fatty acid transporter gene by insulin. Mol Endocrinol 1996; 10: 1021–1028.
Bower JF, Davis JM, Hao E, Barakat HA . Differences in transport of fatty acids and expression of fatty acid transporting proteins in adipose tissue of obese black and white women. Am J Physiol 2006; 290: E87–E91.
Hajri T, Abumrad NA . Fatty acid transport across membranes: relevance to nutrition and metabolic pathology. Annu Rev Nutr 2002; 22: 383–415.
Febbraio M, Abumrad NA, Hajjar DP, Sharma K, Cheng W, Pearce SF et al. A null mutation in murine CD36 reveals an important role in fatty acid and lipoprotein metabolism. J Biol Chem 1999; 274: 19055–19062.
Coburn CT, Knapp Jr FF, Febbraio M, Beets AL, Silverstein RL, Abumrad NA . Defective uptake and utilization of long chain fatty acids in muscle and adipose tissues of CD36 knockout mice. J Biol Chem 2000; 275: 32523–32529.
Pohl J, Ring A, Korkmaz U, Ehehalt R, Stremmel W . FAT/CD36-mediated long-chain fatty acid uptake in adipocytes requires plasma membrane rafts. Mol Biol Cell 2005; 16: 24–31.
Ring A, Le Lay S, Pohl J, Verkade P, Stremmel W . Caveolin-1 is required for fatty acid translocase (FAT/CD36) localization and function at the plasma membrane of mouse embryonic fibroblasts. Biochim Biophys Acta 2006; 1761: 416–423.
Ehehalt R, Fullekrug J, Pohl J, Ring A, Herrmann T, Stremmel W . Translocation of long chain fatty acids across the plasma membrane—lipid rafts and fatty acid transport proteins. Mol Cell Biochem 2006; 284: 135–140.
Kampf JP, Parmley D, Kleinfeld AM . Free fatty acid transport across adipocytes is mediated by an unknown membrane protein pump. Am J Physiol 2007; 293: E1207–E1214.
Gregor MG, Hotamisligil GS . Adipocyte stress: the endoplasmic reticulum and metabolic disease. J Lipid Res 2007; 48: 1905–1914.
Meirhaeghe A, Martin G, Nemoto M, Deeb S, Cottel D, Auwerx J et al. Intronic polymorphism in the fatty acid transport protein 1 gene is associated with increased plasma triglyceride levels in a French population. Arterioscler Thromb Vasc Biol 2000; 20: 1330–1334.
Gertow K, Skoglund-Andersson C, Eriksson P, Boquist S, Orth-Gomer K, Schenck-Gustafsson K et al. A common polymorphism in the fatty acid transport protein-1 gene associated with elevated post-prandial lipaemia and alterations in LDL particle size distribution. Atherosclerosis 2003; 167: 265–273.
Gertow K, Bellanda M, Eriksson P, Boquist S, Hamsten A, Sunnerhagen M et al. Genetic and structural evaluation of fatty acid transport protein-4 in relation to markers of the insulin resistance syndrome. J Clin Endocrinol Metab 2004; 89: 392–399.
Fisher RM, Gertow K . Fatty acid transport proteins and insulin resistance. Curr Opin Lipidol 2005; 16: 173–178.
Gargiulo CE, Stuhlsatz-Krouper SM, Schaffer JE . Localization of adipocyte long-chain fatty acyl-CoA synthetase at the plasma membrane. J Lipid Res 1999; 40: 881–892.
Coleman RA, Lee DP . Enzymes of triacylglycerol synthesis and their regulation. Prog Lipid Res 2004; 43: 134–176.
Smith SJ, Cases S, Jensen DR, Chen HC, Sande E, Tow B et al. Obesity resistance and multiple mechanisms of triglyceride synthesis in mice lacking Dgat. Nat Genet 2000; 25: 87–90.
Cases S, Smith SJ, Zheng YW, Myers HM, Lear SR, Sande E et al. Identification of a gene encoding an acyl CoA:diacylglycerol acyltransferase, a key enzyme in triacylglycerol synthesis. Proc Natl Acad Sci USA 1998; 95: 13018–13023.
Cases S, Stone SJ, Zhou P, Yen E, Tow B, Lardizabal KD et al. Cloning of DGAT2, a second mammalian diacylglycerol acyltransferase, and related family members. J Biol Chem 2001; 276: 38870–38876.
Yen CL, Monetti M, Burri BJ, Farese Jr RV . The triacylglycerol synthesis enzyme DGAT1 also catalyzes the synthesis of diacylglycerols, waxes, and retinyl esters. J Lipid Res 2005; 46: 1502–1511.
Chen HC, Farese Jr RV . Inhibition of triglyceride synthesis as a treatment strategy for obesity: lessons from DGAT1-deficient mice. Arterioscler Thromb Vasc Biol 2005; 25: 482–486.
Chen HC, Jensen DR, Myers HM, Eckel RH, Farese Jr RV . Obesity resistance and enhanced glucose metabolism in mice transplanted with white adipose tissue lacking acyl CoA:diacylglycerol acyltransferase 1. J Clin Invest 2003; 111: 1715–1722.
Chen HC, Stone SJ, Zhou P, Buhman KK, Farese Jr RV . Dissociation of obesity and impaired glucose disposal in mice overexpressing acyl coenzyme a:diacylglycerol acyltransferase 1 in white adipose tissue. Diabetes 2002; 51: 3189–3195.
Ranganathan G, Unal R, Pokrovskaya I, Yao-Borengasser A, Phanavanh B, Lecka-Czernik B et al. The lipogenic enzymes DGAT1, FAS, and LPL in adipose tissue: effects of obesity, insulin resistance, and TZD treatment. J Lipid Res 2006; 47: 2444–2450.
O'Brien RM, Granner DK . Regulation of gene expression by insulin. Physiol Rev 1996; 76: 1109–1161.
Kalant D, Cain SA, Maslowska M, Sniderman AD, Cianflone K, Monk PN . The chemoattractant receptor-like protein C5L2 binds the C3a des-Arg77/acylation-stimulating protein. J Biol Chem 2003; 278: 11123–11129.
Faraj M, Sniderman AD, Cianflone K . ASP enhances in situ lipoprotein lipase activity by increasing fatty acid trapping in adipocytes. J Lipid Res 2004; 45: 657–666.
Maslowska M, Legakis H, Assadi F, Cianflone K . Targeting the signaling pathway of acylation stimulating protein. J Lipid Res 2006; 47: 643–652.
Evans K, Burdge GC, Wootton SA, Clark ML, Frayn KN . Regulation of dietary fatty acid entrapment in subcutaneous adipose tissue and skeletal muscle. Diabetes 2002; 51: 2684–2690.
Beale EG, Hammer RE, Antoine B, Forest C . Glyceroneogenesis comes of age. FASEB J 2002; 16: 1695–1696.
Guan HP, Li Y, Jensen MV, Newgard CB, Steppan CM, Lazar MA . A futile metabolic cycle activated in adipocytes by antidiabetic agents. Nat Med 2002; 8: 1122–1128.
Tan GD, Debard C, Tiraby C, Humphreys SM, Frayn KN, Langin D et al. A ‘futile cycle’ induced by thiazolidinediones in human adipose tissue? Nat Med 2003; 9: 811–812; author reply 812.
Tordjman J, Chauvet G, Quette J, Beale EG, Forest C, Antoine B . Thiazolidinediones block fatty acid release by inducing glyceroneogenesis in fat cells. J Biol Chem 2003; 278: 18785–18790.
Leroyer SN, Tordjman J, Chauvet G, Quette J, Chapron C, Forest C et al. Rosiglitazone controls fatty acid cycling in human adipose tissue by means of glyceroneogenesis and glycerol phosphorylation. J Biol Chem 2006; 281: 13141–13149.
Cadoudal T, Leroyer S, Reis AF, Tordjman J, Durant S, Fouque F et al. Proposed involvement of adipocyte glyceroneogenesis and phosphoenolpyruvate carboxykinase in the metabolic syndrome. Biochimie 2005; 87: 27–32.
Cadoudal T, Blouin JM, Collinet M, Fouque F, Tan GD, Loizon E et al. Acute and selective regulation of glyceroneogenesis and cytosolic phosphoenolpyruvate carboxykinase in adipose tissue by thiazolidinediones in type 2 diabetes. Diabetologia 2007; 50: 666–675.
Horowitz JF . Fatty acid mobilization from adipose tissue during exercise. Trends Endocrinol Metab 2003; 14: 386–392.
Langin D, Lafontan M . Lipolysis and lipid mobilization in human adipose tissue. In: Bray GA, Bouchard C (eds). Handbook of Obesity. Etiology and Pathophysiology, 2nd edn. vol. Marcel Dekker, Inc.: New York, Basel, 2004, pp 515–532.
Langin D, Lucas S, Lafontan M . Millenium fat-cell lipolysis reveals unsuspected novel tracks. Horm Metab Res 2000; 32: 443–452.
Langin D . Adipose tissue lipolysis as a metabolic pathway to define pharmacological strategies against obesity and the metabolic syndrome. Pharmacol Res 2006; 53: 482–491.
Getty L, Panteleon AE, Mittelman SD, Dea MK, Bergman RN . Rapid oscillations in omental lipolysis are independent of changing insulin levels in vivo. J Clin Invest 2000; 106: 421–430.
Karpe F, Fielding BA, Coppack SW, Lawrence VJ, Macdonald IA, Frayn KN . Oscillations of fatty acid and glycerol release from human subcutaneous adipose tissue in vivo. Diabetes 2005; 54: 1297–1303.
Nielsen S, Guo Z, Johnson CM, Hensrud DD, Jensen MD . Splanchnic lipolysis in human obesity. J Clin Invest 2004; 113: 1582–1588.
Brasaemle DL . Thematic review series: adipocyte biology. The perilipin family of structural lipid droplet proteins: stabilization of lipid droplets and control of lipolysis. J Lipid Res 2007; 48: 2547–2559.
Miyoshi H, Perfield II JW, Souza SC, Shen WJ, Zhang HH, Stancheva ZS et al. Control of adipose triglyceride lipase action by serine 517 of perilipin A globally regulates protein kinase A-stimulated lipolysis in adipocytes. J Biol Chem 2007; 282: 996–1002.
Marcinkiewicz A, Gauthier D, Garcia A, Brasaemle DL . The phosphorylation of serine 492 of perilipin a directs lipid droplet fragmentation and dispersion. J Biol Chem 2006; 281: 11901–11909.
Miyoshi H, Souza SC, Zhang HH, Strissel KJ, Christoffolete MA, Kovsan J et al. Perilipin promotes hormone-sensitive lipase-mediated adipocyte lipolysis via phosphorylation-dependent and -independent mechanisms. J Biol Chem 2006; 281: 15837–15844.
Baar RA, Dingfelder CS, Smith LA, Bernlohr DA, Wu C, Lange AJ et al. Investigation of in vivo fatty acid metabolism in AFABP/aP2(−/−) mice. Am J Physiol 2005; 288: E187–E193.
Smith AJ, Sanders MA, Thompson BR, Londos C, Kraemer FB, Bernlohr DA . Physical association between the adipocyte fatty acid-binding protein and hormone-sensitive lipase: a fluorescence resonance energy transfer analysis. J Biol Chem 2004; 279: 52399–52405.
Zimmermann R, Strauss JG, Haemmerle G, Schoiswohl G, Birner-Gruenberger R, Riederer M et al. Fat mobilization in adipose tissue is promoted by adipose triglyceride lipase. Science 2004; 306: 1383–1386.
Villena JA, Roy S, Sarkadi-Nagy E, Kim K-H, Sul HS . Desnutrin, an adipocyte gene encoding a novel patatin domain-containing protein, is induced by fasting and glucocorticoids: ectopic expression of desnutrin increases triglyceride hydrolysis. J Biol Chem 2004; 279: 47066–47075.
Jenkins CM, Mancuso DJ, Yan W, Sims HF, Gibson B, Gross RW . Identification, cloning, expression, and purification of three novel human calcium-independent phospholipase A2 family members possessing triacylglycerol lipase and acylglycerol transacylase activities. J Biol Chem 2004; 279: 48968–48975.
Zechner R, Strauss JG, Haemmerle G, Lass A, Zimmermann R . Lipolysis: pathway under construction. Curr Opin Lipidol 2005; 16: 333–340.
Kershaw EE, Hamm JK, Verhagen LA, Peroni O, Katic M, Flier JS . Adipose triglyceride lipase: function, regulation by insulin, and comparison with adiponutrin. Diabetes 2006; 55: 148–157.
Haemmerle G, Lass A, Zimmermann R, Gorkiewicz G, Meyer C, Rozman J et al. Defective lipolysis and altered energy metabolism in mice lacking adipose triglyceride lipase. Science 2006; 312: 734–737.
Lass A, Zimmermann R, Haemmerle G, Riederer M, Schoiswohl G, Schweiger M et al. Adipose triglyceride lipase-mediated lipolysis of cellular fat stores is activated by CGI-58 and defective in Chanarin-Dorfman Syndrome. Cell Metab 2006; 3: 309–319.
Schweiger M, Schreiber R, Haemmerle G, Lass A, Fledelius C, Jacobsen P et al. Adipose triglyceride lipase and hormone-sensitive lipase are the major enzymes in adipose tissue triacylglycerol catabolism. J Biol Chem 2006; 281: 40236–40241.
Granneman JG, Moore HP, Granneman RL, Greenberg AS, Obin MS, Zhu Z . Analysis of lipolytic protein trafficking and interactions in adipocytes. J Biol Chem 2007; 282: 5726–5735.
Fredrikson G, Tornqvist H, Belfrage P . Hormone-sensitive lipase and monoacylglycerol lipase are both required for complete degradation of adipocyte triacylglycerol. Biochim Biophys Acta 1986; 876: 288–293.
Langin D, Dicker A, Tavernier G, Hoffstedt J, Mairal A, Ryden M et al. Adipocyte lipases and defect of lipolysis in human obesity. Diabetes 2005; 54: 3190–3197.
Mairal A, Langin D, Arner P, Hoffstedt J . Human adipose triglyceride lipase is not regulated by obesity and exhibits low in vitro triglyceride hydrolase activity. Diabetologia 2006; 49: 1629–1636.
Kuriyama H, Kawamoto S, Ishida N, Ohno I, Mita S, Matsuzawa Y et al. Molecular cloning and expression of a novel human aquaporin from adipose tissue with glycerol permeability. Biochem Biophys Res Commun 1997; 241: 53–58.
Kishida K, Kuriyama H, Funahashi T, Shimomura I, Kihara S, Ouchi N et al. Aquaporin adipose, a putative glycerol channel in adipocytes. J Biol Chem 2000; 275: 20896–20902.
Hibuse T, Maeda N, Nagasawa A, Funahashi T . Aquaporins and glycerol metabolism. Biochim Biophys Acta 2006; 1758: 1004–1011.
Rodriguez A, Catalan V, Gomez-Ambrosi J, Fruhbeck G . Role of aquaporin-7 in the pathophysiological control of fat accumulation in mice. FEBS Lett 2006; 580: 4771–4776.
Prudente S, Flex E, Morini E, Turchi F, Capponi D, De Cosmo S et al. A functional variant of the adipocyte glycerol channel aquaporin 7 gene is associated with obesity and related metabolic abnormalities. Diabetes 2007; 56: 1468–1474.
Ceperuelo-Mallafre V, Miranda M, Chacon MR, Vilarrasa N, Megia A, Gutierrez C et al. Adipose tissue expression of the glycerol channel aquaporin-7 gene is altered in severe obesity but not in type 2 diabetes. J Clin Endocrinol Metab 2007; 92: 3640–3645.
Mauriege P, De Pergola G, Berlan M, Lafontan M . Human fat cell beta-adrenergic receptors: beta-agonist-dependent lipolytic responses and characterization of beta-adrenergic binding sites on human fat cell membranes with highly selective beta 1-antagonists. J Lipid Res 1988; 29: 587–601.
Tavernier G, Barbe P, Galitzky J, Berlan M, Caput D, Lafontan M et al. Expression of beta3-adrenoceptors with low lipolytic action in human subcutaneous white adipocytes. J Lipid Res 1996; 37: 87–97.
Lonnqvist F, Thome A, Nilsell K, Hoffstedt J, Arner P . A pathogenic role of visceral fat beta 3-adrenoceptors in obesity. J Clin Invest 1995; 95: 1109–1116.
Schiffelers SL, Blaak EE, Saris WH, van Baak MA . In vivo beta3-adrenergic stimulation of human thermogenesis and lipid use. Clin Pharmacol Ther 2000; 67: 558–566.
Lafontan M, Berlan M . Fat cell alpha 2-adrenoceptors: the regulation of fat cell function and lipolysis. Endocr Rev 1995; 16: 716–738.
Arner P . Catecholamine-induced lipolysis in obesity. Int J Obesity 1999; 23 (Suppl 1): 10–13.
Jensen MD . Lipolysis: contribution from regional fat. Annu Rev Nutr 1997; 17: 127–139.
Stich V, De Glisezinski I, Crampes F, Hejnova J, Cottet-Emard JM, Galitzky J et al. Activation of alpha(2)-adrenergic receptors impairs exercise-induced lipolysis in SCAT of obese subjects. Am J Physiol Regul Integr Comp Physiol 2000; 279: R499–R504.
De Glisezinski I, Marion-Latard F, Crampes F, Berlan M, Hejnova J, Cottet-Emard J-M et al. Lack of alpha2-adrenergic antilipolytic effect during exercise in subcutaneous adipose tissue of trained men. J Appl Physiol 2001; 91: 1760–1765.
Polak J, Moro C, Klimcakova E, Hejnova J, Majercik M, Viguerie N et al. Dynamic strength training improves insulin sensitivity and functional balance between adrenergic alpha 2A and beta pathways in subcutaneous adipose tissue of obese subjects. Diabetologia 2005; 48: 2631–2640.
Richterova B, Stich V, Moro C, Polak J, Klimcakova E, Majercik M et al. Effect of endurance training on adrenergic control of lipolysis in adipose tissue of obese women. J Clin Endocrinol Metab 2004; 89: 1325–1331.
Moro C, Pillard F, de Glisezinski I, Crampes F, Thalamas C, Harant I et al. Sex differences in lipolysis-regulating mechanisms in overweight subjects: effect of exercise intensity. Obesity (Silver Spring) 2007; 15: 2245–2255.
Birkenfeld AL, Boschmann M, Moro C, Adams F, Heusser K, Franke G et al. Lipid mobilization with physiological atrial natriuretic peptide concentrations in humans. J Clin Endocrinol Metab 2005; 90: 3622–3628.
Moro C, Crampes F, Sengenes C, De Glisezinski I, Galitzky J, Thalamas C et al. Atrial natriuretic peptide contributes to physiological control of lipid mobilization in humans. FASEB J 2004; 18: 908–910.
Moro C, Pillard F, de Glisezinski I, Crampes F, Thalamas C, Harant I et al. Atrial natriuretic peptide contribution to lipid mobilization and utilization during head-down bed rest in humans. Am J Physiol Regul Integr Comp Physiol 2007; 293: R612–R617.
Lafontan M, Moro C, Sengenes C, Galitzky J, Crampes F, Berlan M . An unsuspected metabolic role for atrial natriuretic peptides: the control of lipolysis, lipid mobilization, and systemic nonesterified fatty acids levels in humans. Arterioscler Thromb Vasc Biol 2005; 25: 2032–2042.
Langin D, Arner P . Importance of TNFalpha and neutral lipases in human adipose tissue lipolysis. Trends Endocrinol Metab 2006; 17: 314–320.
Kuo LE, Kitlinska JB, Tilan JU, Li L, Baker SB, Johnson MD et al. Neuropeptide Y acts directly in the periphery on fat tissue and mediates stress-induced obesity and metabolic syndrome. Nat Med 2007; 13: 803–811.
Wise A, Foord SM, Fraser NJ, Barnes AA, Elshourbagy N, Eilert M et al. Molecular identification of high and low affinity receptors for nicotinic acid. J Biol Chem 2003; 278: 9869–9874.
Karpe F, Frayn KN . The nicotinic acid receptor—a new mechanism for an old drug. Lancet 2004; 363: 1892–1894.
Taggart AK, Kero J, Gan X, Cai TQ, Cheng K, Ippolito M et al. (D)-beta-Hydroxybutyrate inhibits adipocyte lipolysis via the nicotinic acid receptor PUMA-G. J Biol Chem 2005; 280: 26649–26652.
Carlson LA . Nicotinic acid: the broad-spectrum lipid drug. A 50th anniversary review. J Intern Med 2005.
Benyo Z, Gille A, Kero J, Csiky M, Suchankova MC, Nusing RM et al. GPR109A (PUMA-G/HM74A) mediates nicotinic acid-induced flushing. J Clin Invest 2005; 115: 3634–3640.
Wang T, Zang Y, Ling W, Corkey BE, Guo W . Metabolic partitioning of endogenous fatty acid in adipocytes. Obes Res 2003; 11: 880–887.
Frayn KN, Humphreys SM, Coppack SW . Fuel selection in white adipose tissue. Proc Nutr Soc 1995; 54: 177–189.
Coppack SW, Fisher RM, Gibbons GF, Humphreys SM, McDonough MJ, Potts JL et al. Postprandial substrate deposition in human forearm and adipose tissues in vivo. Clin Sci (London) 1990; 79: 339–348.
Maassen JA, Romijn JA, Heine RJ . Fatty acid-induced mitochondrial uncoupling in adipocytes as a key protective factor against insulin resistance and beta cell dysfunction: a new concept in the pathogenesis of obesity-associated type 2 diabetes mellitus. Diabetologia 2007; 50: 2036–2041.
Frayn KN, Langin D, Karpe F . Fatty acid-induced mitochondrial uncoupling in adipocytes is not a promising target for treatment of insulin resistance unless adipocyte oxidative capacity is increased. Diabetologia 2008; 51: 394–397.
Tiraby C, Tavernier G, Lefort C, Larrouy D, Bouillaud F, Ricquier D et al. Acquirement of brown fat cell features by human white adipocytes. J Biol Chem 2003; 278: 33370–33376.
Mazzucotelli A, Viguerie N, Tiraby C, Annicotte JS, Mairal A, Klimcakova E et al. The transcriptional coactivator peroxisome proliferator activated receptor (PPAR)gamma coactivator-1 alpha and the nuclear receptor PPAR alpha control the expression of glycerol kinase and metabolism genes independently of PPAR gamma activation in human white adipocytes. Diabetes 2007; 56: 2467–2475.
Tiraby C, Langin D . Conversion from white to brown adipocytes: a strategy for the control of fat mass? Trends Endocrinol Metab 2003; 14: 439–441.
Kussmann M, Raymond F, Affolter M . OMICS-driven biomarker discovery in nutrition and health. J Biotechnol 2006; 124: 758–787.
Bourlier V, Zakaroff-Girard A, Miranville A, De Barros S, Maumus M, Sengenes C et al. Remodeling phenotype of human subcutaneous adipose tissue macrophages. Circulation 2008; 117: 806–815.
Acknowledgements
I acknowledge that the following colleagues contributed to the studies on the effects of catecholamine and ANP in human adipose tissue and their contribution to the discussion of the review: Michel Berlan, Anne Bouloumié, François Crampes, Isabelle de Glisezinski, Jean Galitzky, Dominique Langin, Coralie Sengenes, Cédric Moro and Vladimir Stich. Owing to the extent of the topic, I apologize to numerous colleagues for not citing their work because of space limitations.
Author information
Authors and Affiliations
Corresponding author
Additional information
Conflict of interest
The author has declared no financial interests.
Rights and permissions
About this article
Cite this article
Lafontan, M. Advances in adipose tissue metabolism. Int J Obes 32 (Suppl 7), S39–S51 (2008). https://doi.org/10.1038/ijo.2008.237
Published:
Issue Date:
DOI: https://doi.org/10.1038/ijo.2008.237
Keywords
This article is cited by
-
The circadian clock and metabolic homeostasis: entangled networks
Cellular and Molecular Life Sciences (2021)
-
Adipose HuR protects against diet-induced obesity and insulin resistance
Nature Communications (2019)
-
Rapeseed oil fortified with micronutrients can reduce glucose intolerance during a high fat challenge in rats
Nutrition & Metabolism (2018)
-
Microfluidic systems for studying dynamic function of adipocytes and adipose tissue
Analytical and Bioanalytical Chemistry (2018)
-
Formation of a nucleoplasmic reticulum requires de novo assembly of nascent phospholipids and shows preferential incorporation of nascent lamins
Scientific Reports (2017)