Abstract
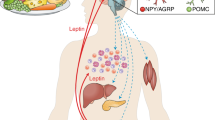
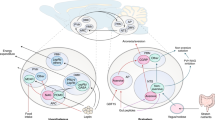
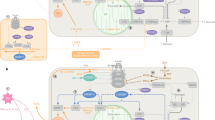
The control of body weight and of blood glucose concentrations depends on the exquisite coordination of the function of several organs and tissues, in particular the liver, muscle and fat. These organs and tissues have major roles in the use and storage of nutrients in the form of glycogen or triglycerides and in the release of glucose or free fatty acids into the blood, in periods of metabolic needs. These mechanisms are tightly regulated by hormonal and nervous signals, which are generated by specialized cells that detect variations in blood glucose or lipid concentrations. The hormones insulin and glucagon not only regulate glycemic levels through their action on these organs and the sympathetic and parasympathetic branches of the autonomic nervous system, which are activated by glucose or lipid sensors, but also modulate pancreatic hormone secretion and liver, muscle and fat glucose and lipid metabolism. Other signaling molecules, such as the adipocyte hormones leptin and adiponectin, have circulating plasma concentrations that reflect the level of fat stored in adipocytes. These signals are integrated at the level of the hypothalamus by the melanocortin pathway, which produces orexigenic and anorexigenic neuropeptides to control feeding behavior, energy expenditure and glucose homeostasis. Work from several laboratories, including ours, has explored the physiological role of glucose as a signal that regulates these homeostatic processes and has tested the hypothesis that the mechanism of glucose sensing that controls insulin secretion by the pancreatic beta-cells is also used by other cell types. I discuss here evidence for these mechanisms, how they integrate signals from other nutrients such as lipids and how their deregulation may initiate metabolic diseases.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout

Similar content being viewed by others
References
Dentin R, Denechaud PD, Benhamed F, Girard J, Postic C . Hepatic gene regulation by glucose and polyunsaturated fatty acids: a role for ChREBP. J Nutr 2006; 136: 1145–1149.
Matschinsky FM . A lesson in metabolic regulation inspired by the glucokinase sensor paradigm. Diabetes 1996; 45: 223–241.
Thorens B . GLUT2 in pancreatic and extra-pancreatic gluco-detection (review). Mol Membr Biol 2001; 18: 265–273.
Inagaki N, Gonoi T, Seino S . Subunit stoichiometry of the pancreatic beta-cell ATP-sensitive K+ channel. FEBS Lett 1997; 409: 232–236.
Thorens B . The hepatoportal glucose sensor. Mechanisms of glucose sensing and signal transduction. In: Matschinski FM, Magnuson, MA (eds). Glucokinase and Glycemic Disease: From Basics to Novel Therapeutics, vol 16. Karger: Basel, 2004. pp 327–338.
Burcelin R, Dolci W, Thorens B . Glucose sensing by the hepatoportal sensor is GLUT2-dependent. In vivo analysis in GLUT 2-null mice. Diabetes 2000; 49: 1643–1648.
Jetton TL, Liang Y, Pettepher CC, Zimmerman EC, Cox FG, Horvath K et al. Analysis of upstream glucokinase promoter activity in transgenic mice and identification of glucokinase in rare neuroendocrine cells in the brain and gut. J Biol Chem 1994; 269: 3641–3654.
Burcelin R, Dolci W, Thorens B . Portal glucose infusion in the mouse induces hypoglycemia. Evidence that the hepatoportal glucose sensor stimulates glucose utilization. Diabetes 2000; 49: 1635–1642.
Burcelin R, DaCosta A, Drucker D, Thorens B . Glucose competence of the hepatoporal vein sensor requires the presence of an activated GLP-1 receptor. Diabetes 2001; 50: 1720–1728.
Ionut V, Hucking K, Liberty IF, Bergman RN . Synergistic effect of portal glucose and glucagon-like peptide-1 to lower systemic glucose and stimulate counter-regulatory hormones. Diabetologia 2005; 48: 967–975.
Johnson KM, Edgerton DS, Rodewald T, Scott M, Farmer B, Neal D et al. Intraportal GLP-1 infusion increases nonhepatic glucose utilization without changing pancreatic hormone levels. Am J Physiol Endocrinol Metab 2007; 293: E1085–E1091.
Berthoud H-R, Neuhuber WL . Functional and chemical anatomy of the afferent vagal system. Auton Neurosci 2000; 85: 1–17.
Niijima A . Afferent impulse discharges from glucoreceptors in the liver of the guinea pig. Ann N Y Acad Sci 1969; 157: 690–700.
Nakabayashi H, Nishizawa M, Nakagawa A, Takeda R, Niijima A . Vagal hepatopancreatic reflex effect evoked by intraportal appearance of tGLP-1. Am J Physiol 1996; 271: E808–E813.
Matveyenko AV, Donovan CM . Metabolic sensors mediate hypoglycemic detection at the portal vein. Diabetes 2006; 55: 1276–1282.
Balkan B, Li X . Portal GLP-1 administration in rats augments the insulin response to glucose via neuronal mechanisms. Am J Physiol 2000; 279: R1449–R1454.
Preitner F, Ibberson M, Franklin I, Binnert C, Pende M, Gjinovici A et al. Gluco-incretin control insulin secretion at multiple levels as revealed in mice lacking GLP-1 and GIP receptors. J Clin Invest 2004; 113: 635–645.
Gardemann A, Strulik H, Jungermann K . A portal-arterial glucose concentration gradient as a signal for an insulin-dependent net glucose uptake in perfused rat liver. FEBS Lett 1986; 202: 255–259.
Burcelin R, Crivelli V, Perrin C, Da Costa A, Mu J, Kahn BB et al. GLUT4, AMP kinase, but not the insulin receptor, are required for hepatoportal glucose sensor-stimulated muscle glucose utilization. J Clin Invest 2003; 111: 1555–1562.
Russek M . Demonstration of the influence of an hepatic glucosensitive mechanism on food-intake. Physiol Behav 1970; 5: 1207–1209.
Russek M . Participation of hepatic glucoreceptors in the control of intake of food. Nature 1963; 197: 79–80.
Oomura Y, Yoshimatsu H . Neural network of glucose monitoring system. J Auton Nerv Syst 1984; 10: 359–372.
Silver IA, Erecinska M . Glucose-induced intracellular ion changes in sugar-sensitive hypothalamic neurons. J Neurophysiol 1998; 79: 1733–1745.
Spanswick D, Smith M, Groppi V, Logam S, Ashford M . Leptin inhibits hypothalamic neurons by activation of ATP-sensitive potassium channels. Nature 1997; 390: 521–525.
Fioramonti X, Lorsignol A, Taupignon A, Penicaud L . A new ATP-sensitive K+ channel-independent mechanism is involved in glucose-excited neurons of mouse arcuate nucleus. Diabetes 2004; 53: 2767–2775.
Penicaud L, Leloup C, Fioramonti X, Lorsignol A, Benani A . Brain glucose sensing: a subtle mechanism. Curr Opin Clin Nutr Metab Care 2006; 9: 458–462.
Mizuno Y, Oomura Y . Glucose responding neurons in the nucleus tractus solitarius of the rat: in vitro studies. Brain Res 1984; 307: 109–116.
Dallaporta M, Himmi T, Perrin J, Orsini J-C . A solitary tract nucleus sensitivity to moderate changes in glucose level. NeuroReport 1999; 10: 1–4.
Yettefti K, Orsini J-C, Perrin J . Characteristics of glycemia-sensitive neurons in the nucleus tractus solitarii: possible involvement in nutritional regulation. Physiol Behav 1997; 61: 93–100.
Briski KP, Marshall ES, Sylvester PW . Effects of estradiol on glucoprivic transactivation of catecholaminergic neurons in the female rat caudal brainstem. Neuroendocrinology 2001; 73: 369–377.
Adachi A, Shimizu N, Oomura Y, Kobashi M . Convergence of hepatoportal glucose-sensitive afferents signal to glucose sensitive units within the nucleus of the solitary tract. Neurosci Lett 1984; 46: 215–218.
Ritter S, Dinh TT . 2-Mercaptoacetate and 2-deoxy-D-glucose induce Fos-like immunoreactivity in rat brain. Brain Res 1994; 641: 111–120.
Pipeleers DG, Schuit FC, Van Schravendijk CFH, Van De Winkel M . Interplay of nutrients and hormones in the regulation of glucagon release. Endocrinology 1985; 117: 817–823.
Olsen HL, Theander S, Bokvist K, Buschard K, Wollheim CB, Gromada J . Glucose stimulates glucagon release in single rat {alpha}-cells by mecha. Endocrinology 2005; 146: 4861–4870.
Gromada J, Franklin I, Wollheim CB . Alpha-cells of the endocrine pancreas: 35 years of research but the enigma remains. Endocr Rev 2007; 28: 84–116.
Gosmanov NR, Szoke E, Israelian Z, Smith T, Cryer PE, Gerich JE et al. Role of the decrement in intraislet insulin for the glucagon response to hypoglycemia in humans. Diabetes Care 2005; 28: 1124–1131.
Taborsky GJ, Ahrén B, Havel PJ . Autonomic mediation of glucagon secretion during hypoglycemia. Implication for impaired alpha cell responses in type 1 diabetes. Diabetes 1998; 47: 995–1005.
Havel PJ, Akpan JO, Curry DL, Stern JS, Gingerich RL, Ahren B . Autonomic control of pancreatic polypeptide and glucagon secretion during neuroglucopenia and hypoglycemia in mice. Am J Physiol 1993; 265: R246–R254.
Cryer PE, Davis SN, Shamoon H . Hypoglycemia in diabetes. Diabetes Care 2003; 26: 1902–1912.
Dunning BE, Gerich JE . The role of alpha-cell dysregulation in fasting and postprandial hyperglycemia in type 2 diabetes and therapeutic implications. Endocr Rev 2007; 28: 253–283.
Hevener AL, Bergman RN, Donovan CM . Novel glucosensor for hypoglycemic detection localized to the portal vein. Diabetes 1997; 46: 1521–1525.
Hevener AL, Bergman RN, Donovan CM . Portal vein afferents are critical for the sympathoadrenal response to hypoglycemia. Diabetes 2000; 49: 8–12.
Donovan CM, Hamilton-Wessler M, Halter JB, Bergman RN . Primacy of liver glucosensors in the sympathetic response to progressive hypoglycemia. Proc Natl Acad Sci USA 1994; 91: 2863–2867.
Hamilton-Wessler M, Bergman RN, Halter JB, Watanabe RM, Donovan CM . The role of liver glucosensors in the integrated sympathetic response induced by deep hypoglycemia in dogs. Diabetes 1994; 43: 1052–1060.
Jackson PA, Pagliassotti MJ, Shiota M, Neal DW, Cardin S, Cherrington AD . Effects of vagal blockade on the counterregulatory response to insulin-induced hypoglycemia in the dog. Am J Physiol 1997; 273: 1178–1188.
Jackson PA, Cardin S, Coffey CS, Neal DW, Allen EJ, Penaloza AR et al. Effect of hepatic denervation on the counterregulatory response to insulin-induced hypoglycemia in the dog. Am J Physiol Endocrinol Metab 2000; 279: 1249–1257.
Cardin S, Jackson PA, Edgerton DS, Neal DW, Coffey CS, Cherrington AD . Effect of vagal cooling on the counterregulatory response to hypoglycemia induced by a low dose of insulin in the conscious dog. Diabetes 2001; 50: 558–564.
Fujita S, Donovan CM . Celiac-superior mesenteric ganglionectomy, but not vagotomy, suppresses the sympathoadrenal response to insulin-induced hypoglycemia. Diabetes 2005; 54: 3258–3264.
Fujita S, Bohland M, Sanchez-Watts G, Watts AG, Donovan CM . Hypoglycemic detection at the portal vein is mediated by capsaicin-sensitive primary sensory neurons. Am J Physiol Endocrinol Metab 2007; 293: E96–E101.
Frizzell RT, Jones EM, Davis SN, Biggers DW, Myers SR, Connolly CC et al. Counterregulation during hypoglycemia is directed by widespread brain regions. Diabetes 1993; 42: 1253–1261.
Borg WP, Sherwin RS, During MJ, Borg MA, Shulman GI . Local ventromedial hypothalamus glucopenia triggers counterregulatory hormone release. Diabetes 1995; 44: 180–184.
Borg MA, Sherwin RS, Borg WP, Tamborlane WV, Shulman GI . Local ventromedial hypothalamus glucose perfusion blocks counterregulation during systemic hypoglycemia in awake rats. J Clin Invest 1997; 99: 361–365.
Ritter RC, Slusser PG, Stone S . Glucoreceptors controlling feeding and blood glucose: location in the hindbrain. Science 1981; 213: 451–453.
Ritter S, Dinh TT, Zhang Y . Localization of hindbrain glucoreceptive sites controlling food intake and blood glucose. Brain Res 2000; 856: 37–47.
Fraley GS, Ritter S . Immunolesion of norepinephrine and epinephrine afferents to medial hypothalamus alters basal and 2-deoxy-D-glucose-induced neuropeptide Y and agouti-gene-related protein messenger ribonucleic acid expression in the arcuate nucleus. Endocrinology 2003; 411: 75–83.
Ritter S, Bugarith K, Dinh TT . Immunotoxic destruction of distinct catecholamine subgroups produces selective impairment of glucoregulatory responses and neuronal activation. J Comp Neurol 2001; 43: 197–216.
DiRocco RJ, Grill HJ . The forebrain is not essential for sympathoadrenal hyperglycemic response to glucoprivation. Science 1979; 204: 1112–1113.
Ritter S, Llewellyn-Smith I, Dinh TT . Subgroups of hindbrain catecholamine neurons are selectively activated by 2-deoxy-D-glucose induced metabolic challenge. Brain Res 1998; 805: 41–54.
Koyama Y, Coker RH, Stone EE, Lacy DB, Jabbour K, Williams PE et al. Evidence that carotid bodies play an important role in glucoregulation in vivo. Diabetes 2000; 49: 1434–1442.
Lopez-Barneo J . Oxygen and glucose sensing by carotid body glomus cells. Curr Opin Neurobiol 2003; 13: 493–499.
Thorens B, Guillam M-T, Beermann F, Burcelin R, Jaquet M . Transgenic reexpression of Glut1 or Glut2 in pancreatic β cells rescues Glut2-null mice from early death and restores normal glucose-stimulated insulin secretion. J Biol Chem 2000; 275: 23751–23758.
Burcelin R, Thorens B . Evidence that extrapancreatic GLUT2-dependent glucose sensors control glucagon secretion. Diabetes 2001; 50: 1282–1289.
Arluison M, Quignon M, Nguyen P, Thorens B, Leloup C, Penicaud L . Distribution and anatomical localization of the glucose transporter 2 (GLUT2) in the adult rat brain—an immunohistochemical study. J Chem Neuroanat 2004; 28: 117–136.
Marty N, Dallaporta M, Foretz M, Emery M, Tarussio D, Bady I et al. Regulation of glucagon secretion by glucose transporter type 2 (glut2) and astrocyte-dependent glucose sensors. J Clin Invest 2005; 115: 3545–3553.
Woods SC, McKay LD . Intraventricular alloxan eliminates feeding elicited by 2-deoxyglucose. Science 1978; 202: 1209–1211.
Sanders NM, Dunn-Meynell AA, Levin BE . Third ventricular alloxan reversibly impairs glucose counterregulatory responses. Diabetes 2004; 53: 1230–1236.
Evans ML, McCrimmon RJ, Flanagan DE, Keshavarz T, Fan X, McNay EC et al. Hypothalamic ATP-sensitive K+ channels play a key role in sensing hypoglycemia and triggering counterregulatory epinephrine and glucagon responses. Diabetes 2004; 53: 2542–2551.
Miki T, Liss B, Minami K, Shiuchi T, Saraya A, Kashima Y et al. ATP-sensitive K+ channels in the hypothalamus are essential for the maintenance of glucose homeostasis. Nat Neurosci 2001; 4: 507–512.
McCrimmon RJ, Evans ML, Fan X, McNay EC, Chan O, Ding Y et al. Activation of ATP-sensitive K+ channels in the ventromedial hypothalamus amplifies counterregulatory hormone responses to hypoglycemia in normal and recurrently hypoglycemic rats. Diabetes 2005; 54: 3169–3174.
Cummings DE, Schwartz MW . Melanocortins and body weight: a tale of two receptors. Nat Genet 2000; 26: 8–9.
Mayer J . Glucostatic mechanism of regulation of food intake. N Engl J Med 1953; 249: 13–16.
Mobbs CV, Isoda F, Makimura H, Mastaitis JW, Mizuno T, Shu IW et al. Impaired glucose signaling as a cause of obesity and the metabolic syndrome: the glucoadipostatic hypothesis. Physiol Behav 2004; 19: 2–23.
Louis-Sylvestre J, Le Magnen J . A fall in blood glucose level precedes meal onset in free feeding rats. Neurosci Biobehav Rev 1980; 4: 13–15.
Campfield LA, Brandon P, Smith FJ . On-line continuous measurement of blood glucose and meal pattern in free-feeding rats: the role of glucose in meal initiation. Brain Res Bull 1985; 14: 615–617.
Novin D, VanderWeele DA, Rezek M . Infusion of 2-deoxy-D-glucose into the hepatic-portal system causes eating: evidence for peripheral glucoreceptors. Science 1973; 181: 858–860.
Miselis RR, Epstein AN . Feeding induced by intracerebroventricular 2-deoxy-D-glucose in the rat. Am J Physiol 1975; 229: 1438–1447.
Berthoud HR, Mogenson GJ . Ingestive behavior after intracerebral and intracerebroventricular infusions of glucose and 2-deoxy-D-glucose. Am J Physiol 1977; 233: R127–R133.
Schmitt M . Influences of hepatic portal receptors on hypothalamic feeding and satiety centers. Am J Physiol 1973; 225: 1089–1095.
Langhans W, Grossmann F, Geary N . Intrameal hepatic-portal infusion of glucose reduces spontaneous meal size in rats. Physiol Behav 2001; 73: 499–507.
Wan HZ, Hulsey MG, Martin RJ . Intracerebroventricular administration of antisense oligodeoxynucleotide against GLUT2 glucose transporter mRNA reduces food intake, body weight change and glucoprivic feeding response in rats. J Nutrit 1998; 128: 287–291.
Bady I, Marty N, Dallaporta M, Emery M, Gyger J, Tarussio D et al. Evidence from glut2-null mice that glucose is a critical physiological regulator of feeding. Diabetes 2006; 55: 988–995.
Leloup C, Arluison M, Lepetit N, Cartier N, Marfaing-Jallat P, Ferré P et al. Glucose transporter 2 (GLUT2): expression in specific brain nuclei. Brain Res 1994; 638: 221–226.
Li B, Xi X, Roane DS, Ryan DH, Martin RJ . Distribution of glucokinase, glucose transporter GLUT2, sulfonylurea receptor-1, glucagon-like peptide-1 receptor and neuropeptide Y messenger RNAs in rat brain by quantitative real time RT-PCR. Brain Res Mol Brain Res 2003; 113: 139–142.
Kang L, Routh VH, Kuzhikandathil EV, Gaspers LD, Levin BE . Physiological and molecular characteristics of rat hypothalamic ventromedial nucleus glucosensing neurons. Diabetes 2004; 53: 549–559.
Arluison M, Quignon M, Thorens B, Leloup C, Penicaud L . Immunocytochemical localization of the glucose transporter 2 (GLUT2) in the adult rat brain. II. Electron microscopic study. J Chem Neuroanat 2004; 28: 137–146.
Garcia Mde L, Millan C, Balmaceda-Aguilera C, Castro T, Pastor P, Montecinos H et al. Hypothalamic ependymal-glial cells express the glucose transporter GLUT2, a protein involved in glucose sensing. J Neurochem 2003; 86: 709–724.
Maekawa F, Toyoda Y, Torii N, Miwa I, Thompson RC, Foster DL et al. Localization of glucokinase-like immunoreactivity in the rat lower brain stem: for possible location of brain glucose-sensing mechanisms. Endocrinology 2000; 141: 375–384.
Ngarmukos C, Baur EL, Kumagai AK . Co-localization of GLUT1 and GLUT4 in the blood–brain barrier of the rat ventromedial hypothalamus. Brain Res 2001; 900: 1–8.
Parton LE, Ye CP, Coppari R, Enriori PJ, Choi B, Zhang CY et al. Glucose sensing by POMC neurons regulates glucose homeostasis and is impaired in obesity. Nature 2007; 449: 228–232.
Minokoshi Y, Alquier T, Furukawa N, Kim YB, Lee A, Xue B et al. AMP-kinase regulates food intake by responding to hormonal and nutrient signals in the hypothalamus. Nature 2004; 428: 569–574.
Claret M, Smith MA, Batterham RL, Selman C, Choudhury AI, Fryer LG et al. AMPK is essential for energy homeostasis regulation and glucose sensing by POMC and AgRP neurons. J Clin Invest 2007; 117: 2325–2336.
Ritter S, Strang M . Fourth ventricular alloxan injection causes feeding but not hyperglycemia in rats. Brain Res 1982; 249: 198–201.
Stein DT, Esser V, Stevenson BE, Lane KE, Whiteside JH, Daniels MB et al. Essentiality of circulating fatty acids for glucose-stimulated insulin secretion in the fasted rat. J Clin Invest 1996; 97: 2728–2735.
Sako Y, Grill VE . A 48-hour lipid infusion in the rat time-dependently inhibits glucose-induced insulin secretion and B cell oxidation through a process likely coupled to fatty acid oxidation. Endocrinology 1990; 127: 1580–1589.
Zhou Y-P, Grill VE . Long-term exposure of rat pancreatic islets to fatty acids inhibits glucose-induced insulin secretion and biosynthesis through a glucose fatty acid cycle. J Clin Invest 1994; 93: 870–876.
Prentki M, Nolan CJ . Islet beta cell failure in type 2 diabetes. J Clin Invest 2006; 116: 1802–1812.
Gremlich S, Bonny C, Waeber G, Thorens B . Fatty acids decrease IDX-1 expression in rat pancreatic islets and reduce GLUT2, glucokinase, insulin, and somatostatin levels. J Biol Chem 1997; 272: 30261–30269.
Eizirik DL, Cardozo AK, Cnop M . The role for endoplasmic reticulum stress in diabetes mellitus. Endocr Rev 2008; 29: 42–61.
Obici S, Feng Z, Morgan K, Stein D, Karkanias G, Rossetti L . Central administration of oleic acid inhibits glucose production and food intake. Diabetes 2002; 51: 271–275.
Obici S, Feng Z, Arduini A, Conti R, Rossetti L . Inhibition of hypothalamic carnitine palmitoyltransferase-1 decreases food intake and glucose production. Nat Med 2003; 9: 756–761.
He W, Lam TK, Obici S, Rossetti L . Molecular disruption of hypothalamic nutrient sensing induces obesity. Nat Neurosci 2006; 9: 227–233.
Lam TK, Pocai A, Gutierrez-Juarez R, Obici S, Bryan J, Aguilar-Bryan L et al. Hypothalamic sensing of circulating fatty acids is required for glucose homeostasis. Nat Med 2005; 11: 320–327.
Pocai A, Lam TK, Gutierrez-Juarez R, Obici S, Schwartz GJ, Bryan J et al. Hypothalamic K(ATP) channels control hepatic glucose production. Nature 2005; 434: 1026–1031.
Shimokawa T, Kumar MV, Lane MD . Effect of a fatty acid synthase inhibitor on food intake and expression of hypothalamic neuropeptides. Proc Natl Acad Sci USA 2002; 99: 66–71.
Wortman MD, Clegg DJ, D’Alessio D, Woods SC, Seeley RJ . C75 inhibits food intake by increasing CNS glucose metabolism. Nat Med 2003; 9: 483–485.
Hu Z, Dai Y, Prentki M, Chohnan S, Lane MD . A role for hypothalamic malonyl-CoA in the control of food intake. J Biol Chem 2005; 280: 39681–39683.
Clement L, Cruciani-Guglielmacci C, Magnan C, Vincent M, Douared L, Orosco M et al. Intracerebroventricular infusion of a triglyceride emulsion leads to both altered insulin secretion and hepatic glucose production in rats. Pflugers Arch 2002; 445: 375–380.
Cruciani-Guglielmacci C, Hervalet A, Douared L, Sanders NM, Levin BE, Ktorza A et al. Beta oxidation in the brain is required for the effects of non-esterified fatty acids on glucose-induced insulin secretion in rats. Diabetologia 2004; 47: 2032–2038.
Wang R, Cruciani-Guglielmacci C, Migrenne S, Magnan C, Cotero VE, Routh VH . Effects of oleic acid on distinct populations of neurons in the hypothalamic arcuate nucleus are dependent on extracellular glucose levels. J Neurophysiol 2006; 95: 1491–1498.
Acknowledgements
I thank Dr Lourdes Mounien for careful reading of the manuscript. The work from my laboratory was supported by grants from the Swiss National Science Foundation, the National Competence Center for Research Frontiers in Genetics and from the Juvenile Diabetes Research Foundation.
Author information
Authors and Affiliations
Corresponding author
Additional information
Conflict of interest
Bernard Thorens has received a grant from the Swiss National Science Foundation.
Rights and permissions
About this article
Cite this article
Thorens, B. Glucose sensing and the pathogenesis of obesity and type 2 diabetes. Int J Obes 32 (Suppl 6), S62–S71 (2008). https://doi.org/10.1038/ijo.2008.208
Published:
Issue Date:
DOI: https://doi.org/10.1038/ijo.2008.208
Keywords
This article is cited by
-
Heart period and blood pressure characteristics in splanchnic arterial occlusion shock-induced collapse
Journal of Clinical Monitoring and Computing (2017)
-
An epigenomic signature of postprandial hyperglycemia in peripheral blood leukocytes
Journal of Human Genetics (2016)
-
Cilnidipine regulates glucose metabolism and levels of high-molecular adiponectin in diet-induced obese mice
Hypertension Research (2013)