Abstract
Objective:
To investigate the influences of red mold rice (RMR) on obesity and related metabolic abnormalities.
Methods and results:
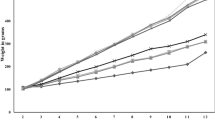
The 3T3-L1 cell line was used to examine the effects of RMR extracts on preadipocytes and on mature adipocytes. Both water and ethanol extracts of RMR had inhibitory effects on 3T3-L1 preadipocyte proliferation and differentiation. Water extracts of RMR enhanced the lipolysis activity in mature adipocytes, which negatively correlated with the triglyceride content within cells. RMR treatment did not affect heparin-releasable lipoprotein lipase activity in mature adipocytes. Furthermore, animal studies were carried out to explore the antiobesity effects of RMR. The control group of male Wistar rats were fed regular laboratory feed, whereas the other groups were fed the high-fat (HF) diet supplemented with lovastatin, rice or RMR (0.4 and 2%, w w−1). The relative caloric intakes of the control and HF groups were 3.34 and 4.85 kcal g−1, respectively. After 6 weeks, rats treated with RMR at the 0.4 and 2% doses had lower weight gain and less fat pads mass accompanied with smaller fat cells than did the HF-diet rats. These effects probably resulted from an increase in the lipolysis activity of adipose tissue and a reduction in food/energy consumption. On the other hand, the RMR supplement significantly reduced serum total cholesterol, serum low-density lipoprotein (LDL) cholesterol, the ratio of LDL to high-density lipoprotein (HDL) cholesterol and serum insulin in the HF group. Moreover, the 2% RMR treatment significantly increased serum HDL cholesterol.
Conclusion:
This study reveals for the first time that RMR can prevent body fat accumulation and improve dyslipidemia. The antiobesity effects of RMR mainly derive from the lipolytic activity and mild antiappetite potency of RMR. In addition, extracts of RMR suppressed the proliferation and differentiation in 3T3-L1 preadipocytes, which might have contributed to the inhibition of new adipocyte formation or hyperplasia in adipose tissue.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Marques BG, Hausman DB, Martin RJ . Association of fat cell size and paracrine growth factors in development of hyperplastic obesity. Am J Physiol 1998; 275: R1898–R1908.
Knittle JL, Timmers K, Ginsbergfellner F, Brown RE, Katz DP . Growth of adipose-tissue in children and adolescents-cross-sectional and longitudinal -studies of adipose cell number and size. J Clin Invest 1979; 63: 239–246.
Kirkland JL, Hollenberg CH, Kindler S, Gillon WS . Effects of age and anatomic site on preadipocyte number in rat fat depots. J Gerontol 1994; 49: B31–B35.
Chumlea WC, Roche AF, Siervogel RM, Knittle JL, Webb P . Adipocytes and adiposity in adults. Am J Clin Nutr 1981; 34: 1798–1803.
Heber D, Yip I, Ashley JM, Elashoff DA, Elashoff RM, Go VLW . Cholesterol-lowering effects of a proprietary Chinese red-yeast-rice dietary supplement. Am J Clin Nutr 1999; 69: 231–236.
Lee CL, Tsai TY, Wang JJ, Pan TM . In vivo hypolipidemic effects and safety of low dosage Monascus powder in a hamster model of hyperlipidemia. Appl Microbiol Biotechnol 2006; 70: 533–540.
Wang JX, Lu ZL, Chi JM, Wang WH, Su MZ, Kou WR et al. Multicenter clinical trial of the serum lipid-lowering effects of a Monascus purpureus (red yeast) rice preparation from traditional Chinese medicine. Curr Ther Res 1997; 58: 964–978.
Hsieh PS, Tai YH . Aqueous extract of Monascus purpureus M9011 prevents and reverses fructose-induced hypertension in rats. J Agric Food Chem 2003; 51: 3945–3950.
Chang JC, Wu MC, Liu IM, Cheng JT . Plasma glucose-lowering action of Hon-Chi in streptozotocin-induced diabetic rats. Horm Metab Res 2006; 38: 76–81.
Chen CC, Liu IM . Release of acetylcholine by Hon-Chi to raise insulin secretion in Wistar rats. Neurosci Lett 2006; 404: 117–121.
Yasukawa K, Takahashi M, Natori S, Yamazaki M, Takeuchi M, Takido M . Azaphilones inhibit tumor promotion by 12-o-tetradecanoylphorbol-13-acetate. Oncology 1994; 45: 108–112.
Lee CL, Wang JJ, Pan TM . Red mold rice extract represses amyloid beta peptide-induced neurotoxicity via potent synergism of anti-inflammatory and anti-oxidative effect. Appl Microb Biotech 2008; 79: 829–841.
Lee CL, Kuo TF, Wang JJ, Pan TM . Red mold rice ameliorates impairment of memory and learning ability in intracerebroventricular amyloid beta-infused rat via repressing amyloid beta accumulation. J Neurosci Res 2007; 85: 3171–3182.
Aniya Y, Yokomakura T, Yonamine M, Shimada K, Nagamine T, Shimabukuro M et al. Screening of antioxidant action of various molds and protection of Monascus anka against experimentally induced liver injuries of rats. Gen Pharmacol 1999; 32: 225–231.
Aniya Y, Ohtani II, Higa T, Miyagi C, Gibo H, Shimabukuro M et al. Dimerumic acid as an antioxidant of the mold: Monascus anka. Free Radi Boil Medic 2000; 28: 999–1004.
Wang JJ, Shieh MJ, Kuo SL, Lee CL, Pan TM . Effect of red mold rice on antifatigue and exercise-related changes in lipid peroxidation in endurance exercise. Appl Microbial Biotechnol 2006; 70: 247–253.
Nakata M, Nagasaka S, Kusaka I, Matsuoka H, Ishibashi S, Yada T . Effects of statins on the adipocyte maturation and expression of glucose transporter 4 (SLC2A4): implications in glycaemic control. Diabetologia 2006; 49: 1881–1892.
Nishio E, Tomiyama K, Nakata H, Watanabe Y . 3-hydroxy-3-methylglutaryl coenzyme A reductase inhibitor impairs cell differentiation in cultured adipogenic cells (3T3-L1). Eur J Pharmacol 1996; 301: 203–206.
Tomiyama K, Nishio E, Watanabe Y . Both wortmannin and simvastatin inhibit the adipogenesis in 3T3-L1 cells during the late phase of differentiation. Japanese J Pharmacol 1999; 80: 375–378.
Jeon T, Hwang SG, Hirai S, Matsui T, Yano H, Kawada T et al. Red yeast rice extracts suppress adipogenesis by down-regulating adipogenic transcription factors and gene expression in 3T3-L1 cells. Life Sci 2004; 75: 3195–3203.
Lee CL, Hung HK, Wang JJ, Pan TM . Improving the ratio of monacolin K to citrinin production of Monascus purpureus NTU 568 under dioscorea medium through the mediation of pH value and ethanol addition. J Agric Food Chem 2007; 55: 6493–6502.
Morgan DM . Tetrazolium (MTT) assay for cellular viability and activity. Methods Mol Biol 1998; 79: 179–183.
Ueno Y, Umemori K, Niimi E, Tanuma S, Nagata S, Sugamata M . Induction of apoptosis by T-2 toxin and other natural toxins in HL-60 human promyelotic leukemia cells. Nat Toxins 1995; 3: 129–137.
Berger JJ, Barnard RJ . Effect of diet on fat cell size and hormone-sensitive lipase activity. Appl Physiol 1999; 87: 227–232.
Kusunoki M, Hara T, Tsutsumi K, Nakamura T, Miyata T, Sakakibara F et al. The lipoprotein lipase activator, NO-1886, suppresses fat accumulation and insulin resistance in rats fed a high-fat diet. Diabetologia 2000; 43: 875–880.
Quinn DM, Shirai K, Jackson RL, Harmony JA . Lipoprotein lipase catalyzed hydrolysis of water-soluble p-nitrophenyl esters. Inhibition by apolipoprotein C-II. Biochemistry 1982; 21: 6872–6879.
Shirai K, Jackson RL . Lipoprotein lipase-catalyzed hydrolysis of p-nitrophenyl butyrate. J Biol Chem 1982; 257: 1253–1258.
Korn ED . Clearing factor, a heparinactivated lipoprotein lipase. I. Isolation and characterization of the enzyme from normal rat heart. J Biol Chem 1955; 215: 1–14.
Fielding CJ . Further characterization of lipoprotein lipase and hepatic postheparin lipase from rat plasma. Biochim Biophys Acta 1972; 280: 569–578.
LaRosa JC, Levy RI, Windmueller HG, Fredrickson DS . Comparison of the triglyceride lipase of liver, adipose tissue, and postheparin plasma. J Lipid Res 1972; 13: 356–363.
Boyd E . The Growth of the Surface Area of Human Body. University of Minnesota Press: Minneapolis, 1935.
Folch J, Lees M, Sloane SGH . A simple method for the isolation and purification of total lipids from animal tissues. J Biol Chem 1957; 226: 497–509.
Friedewald WT, Levy RI, Fredrickson DS . Estimation of the concentration of low-density lipoprotein cholesterol in plasma without use of the preparative ultracentrifuge. Clin Chem 1972; 28: 499–502.
Chen Q, Chan LL, Li ET . Bitter melon (Momordica charantia) reduces adiposity, lowers serum insulin and normalizes glucose tolerance in rats fed a high fat diet. J Nutr 2003; 133: 1088–1093.
Chen HC, Farese RV . Determination of adipocyte size by computer image analysis. J Lipid Res 2002; 43: 986–989.
Soria A, Chicco A, D'Alessandro ME, Rossi A, Lombardo YB . Dietary fish oil reverse epididymal tissue adiposity, cell hypertrophy and insulin resistance in dyslipemic sucrose fed rat model. J Nutr Biochem 2002; 13: 209–218.
Sinzinger H, Mayr F, Schmid P, Granegger S, O'Grady J, Peskar BA . Sleep disturbance and appetite loss after lovastatin. Lancet 1994; 343: 973.
Petit V, Arnould L, Martin P, Monnot MC, Pineau T, Besnard P et al. Chronic high-fat diet affects intestinal fat absorption and postprandial triglyceride levels in the mouse. J Lipid Res 2007; 48: 278–287.
Barnard RJ, Berger JJ, Ong JO, Kern PA . Adipocyte and muscle changes in response to diet leading to obesity. Diabetes Res 1998; 33: 213–228.
Reaven GM . Effects of differences in amount and kind of dietary carbohydrate on plasma glucose and insulin responses in man. Am J Clin Nutr 1979; 32: 2568–2578.
Storlien LH, James DE, Burleigh KM, Chisholm DJ, Kraegen EW . Fat feeding causes widespread in vivoinsulin resistance, decreased energy expenditure, and obesity inrats. Am J Physiol 1986; 251 (Endocrinol Metab): E576–E583.
Barnard RJ, Roberts CK, Varon SM, Berger JJ . Diet-induced insulin resistance precedes other aspects of the metabolic syndrome. J Appl Physiol 1998; 84: 1311–1315.
DeFronzo RA, Ferrannini E . Insulin resistance. A multifaceted syndrome responsible for NIDDM, obesity, hypertension, dyslipidemia, and atherosclerotic cardiovascular disease. Diabetes Care 1991; 14: 173–194.
Endo A . Monacolin K, a new hypocholesterolemic agent produced by a Monascus species. J Antibiot 1979; 32: 852–854.
Su YC, Wang JJ, Lin TT, Pan TM . Production of the secondary metabolites gamma-aminobutyric acid and monacolin K by Monascus. J Ind Microbiol Biotechnol 2003; 30: 41–46.
Akihisa T, Tokuda H, Yasukawa K, Ukiya M, Kiyota A, Sakamoto N et al. Azaphilones, furanoisophthalides, and amino acids from the extracts of Monascus pilosus-fermented rice (red-mold rice) and their chemopreventive effects. J Agric Food Chem 2005; 53: 562–565.
Marek J, Jakub G . Potential antitumor effects of statins (review). Int J Oncol 2003; 23: 1055–1069.
Huang HL, Hong YW, Wong YH, Chen YN, Chyuan JH, Huang CJ et al. Bitter melon (Momordica charantia L.) inhibits adipocyte hypertrophy and down regulates lipogenic gene expression in adipose tissue of diet-induced obese rats. Br J Nutr 2007; 26: 1–10.
Vogels N, Nijs IM, Westerterp-Plantenga MS . The effect of grape-seed extract on 24 h energy intake in humans. Eur J Clin Nutr 2004; 58: 667–673.
Bhattacharya A, Rahman MM, McCarter R, O'Shea M, Fernandes G . Conjugated linoleic acid and chromium lower body weight and visceral fat mass in high-fat-diet-fed mice. Lipids 2006; 41: 437–444.
Acknowledgements
We thank Professor Fuu Sheu (Horticulture Institute, National Taiwan University) for kindly donating the 3T3-L1 cell line.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Chen, WP., Ho, BY., Lee, CL. et al. Red mold rice prevents the development of obesity, dyslipidemia and hyperinsulinemia induced by high-fat diet. Int J Obes 32, 1694–1704 (2008). https://doi.org/10.1038/ijo.2008.156
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ijo.2008.156
Keywords
This article is cited by
-
Metabolic syndrome in adult male rats induced by feeding beef tallow-enriched homemade diet with fructose-containing drinking water
Comparative Clinical Pathology (2021)
-
Preventive effect of Elateriospermum tapos seed extract against obese Sprague Dawley rats
Advances in Traditional Medicine (2020)
-
Lipectomy associated to obesity produces greater fat accumulation in the visceral white adipose tissue of female compared to male rats
Lipids in Health and Disease (2019)
-
Germinated brown rice ameliorates obesity in high-fat diet induced obese rats
BMC Complementary and Alternative Medicine (2016)
-
The advantages of deep ocean water for the development of functional fermentation food
Applied Microbiology and Biotechnology (2015)