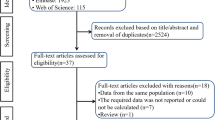
Abstract
The objective of this study is to evaluate the association between chronic periodontitis (CP) and the risk of erectile dysfunction (ED). Electronic search using PubMed, Embase and the Cochrane Library was carried out for observational studies, longitudinal, cohort, case–control and epidemiological studies on humans, published up to December 2015. Manual searches were also performed. Odds ratios (ORs) and corresponding 95% confidence intervals (CIs) were used to estimate the association between CP and the risk of ED. Methodological quality assessment was carried out using the Newcastle-Ottawa Quality Assessment Scale. Four case–control studies and one cross-sectional studies involving 213, 006 participants were included. Based on the random-effects model, analyses of all studies showed that CP was associated with an increased risk of ED (OR=2.28, 95% CI: 1.50–3.48). There was heterogeneity among the studies (P<0.001, I2=97.8%). Estimates of total effects were generally consistent with the sensitivity and subgroup analyses. In conclusion, our meta-analysis suggested that there was a significant association between CP and the risk of ED. Further epidemiological studies are needed to better estimate the key risk factors for periodontitis and their interaction effects.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 8 print issues and online access
$259.00 per year
only $32.38 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
Lue TF . Erectile dysfunction. N Engl J Med 2000; 342: 1802–1813.
Laumann EO, Paik A, Rosen RC . Sexual dysfunction in the United States: prevalence and predictors. JAMA 1999; 281: 537–544.
McCabe MP, Althof SE . A systematic review of the psychosocial outcomes associated with erectile dysfunction: does the impact of erectile dysfunction extend beyond a man's inability to have sex? J Sex Med 2014; 11: 347–363.
Heidelbaugh JJ . Management of erectile dysfunction. Am Fam Physician 2010; 81: 305–312.
Pihlstrom BL, Michalowicz BS, Johnson NW . Periodontal diseases. Lancet 2005; 366: 1809–1820.
Petersen PE, Ogawa H . Strengthening the prevention of periodontal disease: the WHO approach. J Periodontol 2005; 76: 2187–2193.
Fedele S, Sabbah W, Donos N, Porter S, D'Aiuto F . Common oral mucosal diseases, systemic inflammation, and cardiovascular diseases in a large cross-sectional US survey. Am Heart J 2011; 161: 344–350.
Romagna C, Dufour L, Troisgros O, Lorgis L, Richard C, Buffet P et al. Periodontal disease: a new factor associated with the presence of multiple complex coronary lesions. J Clin Periodontol 2012; 39: 38–44.
You Z, Cushman M, Jenny NS, Howard G . Tooth loss, systemic inflammation, and prevalent stroke among participants in the reasons for geographic and racial difference in stroke (REGARDS) study. Atherosclerosis 2009; 203: 615–619.
Pastuszak AW, Hyman DA, Yadav N, Godoy G, Lipshultz LI, Araujo AB et al. Erectile dysfunction as a marker for cardiovascular disease diagnosis and intervention: a cost analysis. J Sex Med 2015; 12: 975–984.
Tonetti MS, D'Aiuto F, Nibali L, Donald A, Storry C, Parkar M et al. Treatment of periodontitis and endothelial function. N Engl J Med 2007; 356: 911–920.
Eltas A, Oguz F, Uslu MO, Akdemir E . The effect of periodontal treatment in improving erectile dysfunction: a randomized controlled trial. J Clin Periodontol 2013; 40: 148–154.
Zuo Z, Jiang J, Jiang R, Chen F, Liu J, Yang H et al. Effect of periodontitis on erectile function and its possible mechanism. J Sex Med 2011; 8: 2598–2605.
Moher D, Liberati A, Tetzlaff J, Altman DG . Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med 2009; 6: e1000097.
Stang A . Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol 2010; 25: 603–605.
DerSimonian R, Laird N . Meta-analysis in clinical trials. Control Clin Trials 1986; 7: 177–188.
Higgins JP, Thompson SG . Quantifying heterogeneity in a meta-analysis. Stat Med 2002; 21: 1539–1558.
Zadik Y, Bechor R, Galor S, Justo D, Heruti RJ . Erectile dysfunction might be associated with chronic periodontal disease: two ends of the cardiovascular spectrum. J Sex Med 2009; 6: 1111–1116.
Keller JJ, Chung SD, Lin HC . A nationwide population-based study on the association between chronic periodontitis and erectile dysfunction. J Clin Periodontol 2012; 39: 507–512.
Oguz F, Eltas A, Beytur A, Akdemir E, Uslu MO, Gunes A . Is there a relationship between chronic periodontitis and erectile dysfunction? J Sex Med 2013; 10: 838–843.
Matsumoto S, Matsuda M, Takekawa M, Okada M, Hashizume K, Wada N et al. Association of ED with chronic periodontal disease. Int J Impot Res 2014; 26: 13–15.
Tsao CW, Liu CY, Cha TL, Wu ST, Chen SC, Hsu CY . Exploration of the association between chronic periodontal disease and erectile dysfunction from a population-based view point. Andrologia 2015; 47: 513–518.
Higashi Y, Goto C, Jitsuiki D, Umemura T, Nishioka K, Hidaka T et al. Periodontal infection is associated with endothelial dysfunction in healthy subjects and hypertensive patients. Hypertension 2008; 51: 446–453.
Sharma A, Pradeep AR, Raju PA . Association between chronic periodontitis and vasculogenic erectile dysfunction. J Periodontol 2011; 82: 1665–1669.
Wang Q, Kang J, Cai X, Wu Y, Zhao L . The association between chronic periodontitis and vasculogenic erectile dysfunction: a systematic review and meta-analysis. J Clin Periodontol 2016; 43: 206–215.
Wu T, Trevisan M, Genco RJ, Falkner KL, Dorn JP, Sempos CT . Examination of the relation between periodontal health status and cardiovascular risk factors: serum total and high density lipoprotein cholesterol, C-reactive protein, and plasma fibrinogen. Am J Epidemiol 2000; 151: 273–282.
Higashi Y, Sasaki S, Nakagawa K, Matsuura H, Oshima T, Chayama K . Endothelial function and oxidative stress in renovascular hypertension. N Engl J Med 2002; 346: 1954–1962.
Bretz WA, Weyant RJ, Corby PM, Ren D, Weissfeld L, Kritchevsky SB et al. Systemic inflammatory markers, periodontal diseases, and periodontal infections in an elderly population. J Am Geriatr Soc 2005; 53: 1532–1537.
Villar IC, Francis S, Webb A, Hobbs AJ, Ahluwalia A . Novel aspects of endothelium-dependent regulation of vascular tone. Kidney Int 2006; 70: 840–853.
Barassi A, Pezzilli R, Colpi GM, Corsi RM, Melzi DG . Vitamin D and erectile dysfunction. J Sex Med 2014; 11: 2792–2800.
Garcia MN, Hildebolt CF, Miley DD, Dixon DA, Couture RA, Spearie CL et al. One-year effects of vitamin D and calcium supplementation on chronic periodontitis. J Periodontol 2011; 82: 25–32.
Kellesarian SV, Kellesarian TV, Ros MV, Al-Askar M, Ghanem A, Malmstrom H et al. Association between periodontal disease and erectile dysfunction: a systematic review. Am J Mens Health 2016 e-pub ahead of print, 29 March 2016 doi:10.1177/1557988316639050.
Nesse W, Dijkstra PU, Abbas F, Spijkervet FK, Stijger A, Tromp JA et al. Increased prevalence of cardiovascular and autoimmune diseases in periodontitis patients: a cross-sectional study. J Periodontol 2010; 81: 1622–1628.
Kalka D, Domagala Z, Rakowska A, Womperski K, Franke R, Sylwina-Krauz E et al. Modifiable risk factors for erectile dysfunction: an assessment of the awareness of such factors in patients suffering from ischaemic heart disease. Int J Impot Res 2016; 28: 14–19.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Rights and permissions
About this article
Cite this article
Liu, L., Li, E., Zhong, S. et al. Chronic periodontitis and the risk of erectile dysfunction: a systematic review and meta-analysis. Int J Impot Res 29, 43–48 (2017). https://doi.org/10.1038/ijir.2016.43
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ijir.2016.43
This article is cited by
-
Evaluation of bi-directional causal association between periodontal disease and erectile dysfunction: a two-sample Mendelian randomization study
Clinical Oral Investigations (2023)
-
The link between periodontitis and erectile dysfunction: a review
British Dental Journal (2019)
-
Chronic periodontitis and the risk of erectile dysfunction: a systematic review and meta-analysis: methodological issues
International Journal of Impotence Research (2017)