Abstract
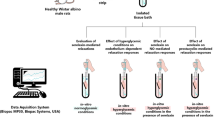
Thiazolidinediones (TZDs) improve vascular endothelial dysfunction through non-genomic effects of peroxisomal proliferator-activated receptor γ. This study investigated the acute effect of one of the TZD, rosiglitazone, on endothelium-dependent relaxation response of corpus cavernosum (CC) in hypercholesterolemic rabbits. New Zealand rabbits were divided into two groups randomly as control and cholesterol groups. Hypercholesterolemia was induced by feeding rabbits with 2% cholesterol diet (w/w) for 6 weeks. Endothelium-dependent and -independent relaxation response of CC were evaluated in the presence of rosiglitazone by organ bath studies with cumulative doses of acetylcholine (Ach) and sodium nitroprusside (SNP). Maximal relaxation (Emax) response to Ach significantly decreased owing to hypercholesterolemia in CC tissues. However, in vitro incubation of rosiglitazone with different concentrations (0.1, 1 and 10 μm) did not improve the Ach-dependent Emax responses in hypercholesterolemic rabbit CC. Surprisingly, rosiglitazone caused a significant decrease in Ach-dependent relaxation in healthy CC. Emax responses to SNP did not differ in the presence of rosiglitazone in both the control and hypercholesterolemic groups. Rosiglitazone does not improve hypercholesterolemia-induced endothelial dysfunction in CC tissues while it dose-dependently impairs endothelium-dependent relaxation in healthy CC tissue.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 8 print issues and online access
$259.00 per year
only $32.38 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Bivalacqua TJ, Usta MF, Champion HC, Kadowitz PJ, Hellstrom WJG . Endothelial dysfunction in erectile dysfunction: role of the endothelium in erectile physiology and disease. J Androl 2003; 24: S17–S37.
Castela A, Vendeira P, Costa C . Testosterone, endothelial health, and erectile function. ISRN Endocrinol 2011; 2011: 839149.
Xie D, Annex BH, Donatucci CF . Growth factors for therapeutic angiogenesis in hypercholesterolemic erectile dysfunction. Asian J Androl 2008; 10: 23–27.
Fraga-Silva RA, Costa-Fraga FP, Faye Y, Sturny M, Santos RA, da Silva RF et al. An increased arginase activity is associated with corpus cavernosum impairment induced by hypercholesterolemia. J Sex Med 2014; 11: 1173–1181.
Liu H-R, Tao L, Gao E, Qu Y, Lau WB, Lopez BL et al. Rosiglitazone inhibits hypercholesterolaemia-induced myeloperoxidase upregulation—a novel mechanism for the cardioprotective effects of PPAR agonists. Cardiovasc Res 2009; 81: 344–352.
Tao L, Liu H-R, Gao E, Teng Z-P, Lopez BL, Christopher TA et al. Antioxidative, antinitrative, and vasculoprotective effects of a peroxisome proliferator–activated receptor-γ agonist in hypercholesterolemia. Circulation 2003; 108: 2805–2811.
Berger JP, Akiyama TE, Meinke PT . PPARs: therapeutic targets for metabolic disease. Trends Pharmacol Sci 2005; 26: 244–251.
Mather KJ . The vascular endothelium in diabetes—a therapeutic target? Rev Endocr Metab Disord 2013; 14: 87–99.
Gholamine B, Shafiei M, Motevallian M, Mahmoudian M . Effects of pioglitazone on erectile dysfunction in sildenafil poor-responders: a randomized, controlled study. J Pharm Pharmaceut Sci 2008; 11: 22–31.
Elcioglu HK, Kabasakal L, Ozkan N, Celikel C, Ayanoglu-Dulger G . A study comparing the effects of rosiglitazone and/or insulin treatments on streptozotocin induced diabetic (type I diabetes) rat aorta and cavernous tissues. Eur J Pharmacol 2011; 660: 476–484.
Soner BC, Murat N, Demir O, Guven H, Esen A, Gidener S . Evaluation of vascular smooth muscle and corpus cavernosum on hypercholesterolemia. Is resveratrol promising on erectile dysfunction[quest]. Int J Impot Res 2010; 22: 227–233.
Mendizabal Y, Llorens S, Nava E . Effects of pioglitazone and rosiglitazone on vascular function of mesenteric resistance arteries in rat genetic hypertension. Pharmacology 2011; 88: 72–81.
Bivalacqua TJ, Usta MF, Champion HC, Kadowitz PJ, Hellstrom WJ . Endothelial dysfunction in erectile dysfunction: role of the endothelium in erectile physiology and disease. J Androl 2003; 24: S17–S37.
Shukla N, Jones R, Persad R, Angelini GD, Jeremy JY . Effect of sildenafil citrate and a nitric oxide donating sildenafil derivative, NCX 911, on cavernosal relaxation and superoxide formation in hypercholesterolaemic rabbits. Eur J Pharmacol 2005; 517: 224–231.
Behr-Roussel D, Bernabe J, Compagnie S, Rupin A, Verbeuren TJ, Alexandre L et al. Distinct mechanisms implicated in atherosclerosis-induced erectile dysfunction in rabbits. Atherosclerosis 2002; 162: 355–362.
Burnett AL, Musicki B, Jin L, Bivalacqua TJ . Nitric oxide/redox-based signalling as a therapeutic target for penile disorders. Expert Opin Ther Targets 2006; 10: 445–457.
Yki-Jarvinen H . Thiazolidinediones. N Engl J Med 2004; 351: 1106–1118.
Kovanecz I, Ferrini MG, Vernet D, Nolazco G, Rajfer J, Gonzalez-Cadavid NF . Ageing-related corpora veno-occlusive dysfunction in the rat is ameliorated by pioglitazone. BJU Int 2007; 100: 867–874.
Gurnell M . PPARgamma and metabolism: insights from the study of human genetic variants. Clin Endocrinol 2003; 59: 267–277.
Aliperti LA, Lasker GF, Hagan SS, Hellstrom JA, Gokce A, Trost LW et al. Efficacy of pioglitazone on erectile function recovery in a rat model of cavernous nerve injury. Urology 2014; 84: 1122–1127.
Aliperti LA, Hellstrom WJ . Pioglitazone prevents cavernosal nerve injury after radical prostatectomy. Med Hypotheses 2014; 82: 504.
Kovanecz I, Ferrini MG, Vernet D, Nolazco G, Rajfer J, Gonzalez-Cadavid NF . Pioglitazone prevents corporal veno-occlusive dysfunction in a rat model of type 2 diabetes mellitus. BJU Int 2006; 98: 116–124.
Ryan MJ, Didion SP, Mathur S, Faraci FM, Sigmund CD . PPAR(gamma) agonist rosiglitazone improves vascular function and lowers blood pressure in hypertensive transgenic mice. Hypertension 2004; 43: 661–666.
Tao L, Liu HR, Gao E, Teng ZP, Lopez BL, Christopher TA et al. Antioxidative, antinitrative, and vasculoprotective effects of a peroxisome proliferator-activated receptor-gamma agonist in hypercholesterolemia. Circulation 2003; 108: 2805–2811.
Zhao Z, Luo Z, Wang P, Sun J, Yu H, Cao T et al. Rosiglitazone restores endothelial dysfunction in a rat model of metabolic syndrome through PPARgamma- and PPARdelta-dependent phosphorylation of Akt and eNOS. PPAR Res 2011; 2011: 291656.
Yang XH, Li P, Yin YL, Tu JH, Dai W, Liu LY et al. Rosiglitazone via PPARgamma-dependent suppression of oxidative stress attenuates endothelial dysfunction in rats fed homocysteine thiolactone. J Cell Mol Med 2015; 19: 826–835.
Majithiya JB, Paramar AN, Balaraman R . Pioglitazone, a PPARgamma agonist, restores endothelial function in aorta of streptozotocin-induced diabetic rats. Cardiovasc Res 2005; 66: 150–161.
Llorens S, Mendizabal Y, Nava E . Effects of pioglitazone and rosiglitazone on aortic vascular function in rat genetic hypertension. Eur J Pharmacol 2007; 575: 105–112.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Rights and permissions
About this article
Cite this article
Akdag, H., Murat, N., Evcim, S. et al. Acute effect of rosiglitazone on relaxation responses in hypercholesterolemic corpus cavernosum. Int J Impot Res 28, 110–113 (2016). https://doi.org/10.1038/ijir.2016.11
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ijir.2016.11