Abstract
It is generally accepted that premature ejaculation (PE) is a more common problem than erectile dysfunction, although at present the options currently available for the treatment of PE are limited to behavioural psychotherapy and ‘off-label’ prescribing of pharmacological therapies. A sexual complaint with such a high prevalence together with an increasing understanding of the psychosocial consequences of PE has naturally stimulated the interest of the pharmaceutical industry and the first products designed specifically for the treatment of PE are either in late-stage clinical development or are already under regulatory review. Most of the new treatments for PE have been developed for ‘on-demand’ use, which may prove to offer the most favourable risk: benefit profile as well as the flexibility to adapt to differing frequencies of sexual activity. This paper reviews a number of emerging therapies in various stages of development that show potential for use in the treatment of PE.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 8 print issues and online access
$259.00 per year
only $32.38 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout
Similar content being viewed by others
References
Waldinger MD . Lifelong premature ejaculation: from authority-based to evidence-based medicine. BJU Int 2004; 93: 201–207.
McMahon CG . Clinical trial methodology in premature ejaculation observational, interventional, and treatment preference studies—part I—defining and selecting the study population. J Sex Med 2008; 5: 1805–1816.
McMahon CG . Clinical trial methodology in premature ejaculation observational, interventional, and treatment preference studies—part II—study design, outcome measures, data analysis, and reporting. J Sex Med 2008; 5: 1817–1833.
Schapiro B . Premature ejaculation: a review of 1130 cases. J Urol 1943; 50: 374–379.
Waldinger MD . Premature ejaculation: definition and drug treatment. Drugs 2007; 67: 547–568.
McMahon CG, Althof S, Waldinger MD, Porst H, Dean J, Sharlip I et al. An evidence-based definition of lifelong premature ejaculation: report of the International Society for Sexual Medicine ad hoc Committee for the Definition of Premature Ejaculation. BJU Int 2008; 102: 338–350.
McMahon CG, Althof SE, Waldinger MD, Porst H, Dean J, Sharlip ID et al. An evidence-based definition of lifelong premature ejaculation: report of the International Society for Sexual Medicine (ISSM) ad hoc Committee for the Definition of Premature Ejaculation. J Sex Med 2008; 5: 1590–1606.
Laumann EO, Paik A, Rosen RC . Sexual dysfunction in the United States: prevalence and predictors. JAMA 1999; 281: 537–544.
Rowland D, Cooper S, Macias L . Pharmaceutical companies could serve their own interests by supporting research on the efficacy of psychotherapy on premature ejaculation. Int J Impot Res 2008; 20: 115–120.
Waldinger MD, Schweitzer DH . Premature ejaculation and pharmaceutical company-based medicine: the dapoxetine case. J Sex Med 2008; 5: 966–997.
Wyllie MG . Another year, another AUA. BJU Int 2006; 97: 1119–1120.
Althof SE, Symonds T . Patient reported outcomes used in the assessment of premature ejaculation. Urol Clin North Am 2007; 34: 581–589, vii.
Althof S, Rosen R, Symonds T, Mundayat R, May K, Abraham L . Development and validation of a new questionnaire to assess sexual satisfaction, control, and distress associated with premature ejaculation. J Sex Med 2006; 3: 465–475.
Symonds T, Perelman MA, Althof S, Giuliano F, Martin M, May K et al. Development and validation of a premature ejaculation diagnostic tool. Eur Urol 2007; 52: 565–573.
Symonds T, Perelman M, Althof S, Giuliano F, Martin M, Abraham L et al. Further evidence of the reliability and validity of the premature ejaculation diagnostic tool. Int J Impot Res 2007; 19: 521–525.
Waldinger MD, Zwinderman AH, Olivier B, Schweitzer DH . The majority of men with lifelong premature ejaculation prefer daily drug treatment: an observation study in a consecutive group of Dutch men. J Sex Med 2007; 4 (4 Part 1): 1028–1037.
Kim JJ, Kwak TI, Jeon BG, Cheon J, Moon DG . Effects of glans penis augmentation using hyaluronic acid gel for premature ejaculation. Int J Impot Res 2004; 16: 547–551.
Kwak TI, Jin MH, Kim JJ, Moon DG . Long-term effects of glans penis augmentation using injectable hyaluronic acid gel for premature ejaculation. Int J Impot Res 2008; 20: 425–428.
Montague DK, Jarow J, Broderick GA, Dmochowski RR, Heaton JP, Lue TF et al. AUA guideline on the pharmacologic management of premature ejaculation. J Urol 2004; 172: 290–294.
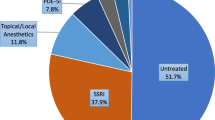
Shindel A, Nelson C, Brandes S . Urologist practice patterns in the management of premature ejaculation: a nationwide survey. J Sex Med 2008; 5: 199–205.
Carani C, Isidori AM, Granata A, Carosa E, Maggi M, Lenzi A et al. Multicenter study on the prevalence of sexual symptoms in male hypo- and hyperthyroid patients. J Clin Endocrinol Metab 2005; 90: 6472–6479.
Shamloul R, el-Nashaar A . Chronic prostatitis in premature ejaculation: a cohort study in 153 men. J Sex Med 2006; 3: 150–154.
Xin ZC, Chung WS, Choi YD, Seong DH, Choi YJ, Choi HK . Penile sensitivity in patients with primary premature ejaculation. J Urol 1996; 156: 979–981.
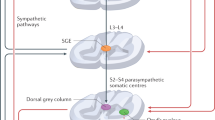
Waldinger MD . The neurobiological approach to premature ejaculation. J Urol 2002; 168: 2359–2367.
Waldinger MD, Rietschel M, Nothen MM, Hengeveld MW, Olivier B . Familial occurrence of primary premature ejaculation. Psychiatr Genet 1998; 8: 37–40.
Giuliano F, Clement P . Physiology of ejaculation: emphasis on serotonergic control. Eur Urol 2005; 48: 408–417.
Giuliano FA, Rampin O, Benoit G, Jardin A . Neural control of penile erection. Urol Clin North Am 1995; 22: 747–766.
Giuliano F, Clement P . Serotonin and premature ejaculation: from physiology to patient management. Eur Urol 2006; 50: 454–466.
Waldinger MD, Berendsen HH, Blok BF, Olivier B, Holstege G . Premature ejaculation and serotonergic antidepressants-induced delayed ejaculation: the involvement of the serotonergic system. Behav Brain Res 1998; 92: 111–118.
Giuliano F . 5-Hydroxytryptamine in premature ejaculation: opportunities for therapeutic intervention. Trends Neurosci 2007; 30: 79–84.
Gur E, Lerer B, Newman ME . Chronic clomipramine and triiodothyronine increase serotonin levels in rat frontal cortex in vivo: relationship to serotonin autoreceptor activity. J Pharmacol Exp Ther 1999; 288: 81–87.
Kim SC, Seo KK . Efficacy and safety of fluoxetine, sertraline and clomipramine in patients with premature ejaculation: a double-blind, placebo controlled study. J Urol 1998; 159: 425–427.
Althof SE, Levine SB, Corty EW, Risen CB, Stern EB, Kurit DM . A double-blind crossover trial of clomipramine for rapid ejaculation in 15 couples. J Clin Psychiatry 1995; 56: 402–407.
Segraves RT, Saran A, Segraves K, Maguire E . Clomipramine versus placebo in the treatment of premature ejaculation: a pilot study. J Sex Marital Ther 1993; 19: 198–200.
Haensel SM, Rowland DL, Kallan KT . Clomipramine and sexual function in men with premature ejaculation and controls. J Urol 1996; 156: 1310–1315.
Strassberg DS, de Gouveia Brazao CA, Rowland DL, Tan P, Slob AK . Clomipramine in the treatment of rapid (premature) ejaculation. J Sex Marital Ther 1999; 25: 89–101.
Waldinger MD, Zwinderman AH, Olivier B . On-demand treatment of premature ejaculation with clomipramine and paroxetine: a randomized, double-blind fixed-dose study with stopwatch assessment. Eur Urol 2004; 46: 510–515; discussion 516.
Leaker B . Abstract P-05-048: a double blind, placebo-controlled, randomised crossover study to investigate the effect of inhaled doses of VR776 on intravaginal ejaculatory latency in patients with premature ejaculation. J Sex Med 2008; 5 (Suppl 2): 60.
Ahlenius S, Larsson K, Svensson L . Further evidence for an inhibitory role of central 5-HT in male rat sexual behavior. Psychopharmacology (Berl) 1980; 68: 217–220.
Wang WF, Chang L, Minhas S, Ralph DJ . Selective serotonin reuptake inhibitors in the treatment of premature ejaculation. Chin Med J (Engl) 2007; 120: 1000–1006.
Waldinger MD, Zwinderman AH, Schweitzer DH, Olivier B . Relevance of methodological design for the interpretation of efficacy of drug treatment of premature ejaculation: a systematic review and meta-analysis. Int J Impot Res 2004; 16: 369–381.
Lue TF, Giuliano F, Montorsi F, Rosen RC, Andersson KE, Althof S et al. Summary of the recommendations on sexual dysfunctions in men. J Sex Med 2004; 1: 6–23.
Lane R, Baldwin D . Selective serotonin reuptake inhibitor-induced serotonin syndrome: review. J Clin Psychopharmacol 1997; 17: 208–221.
Tamam L, Ozpoyraz N . Selective serotonin reuptake inhibitor discontinuation syndrome: a review. Adv Ther 2002; 19: 17–26.
FDA. FDA proposes new warnings about suicidal thinking, behavior in young adults who take antidepressant medications. FDA News 2007.
Giuliano F, Hellstrom WJ . The pharmacological treatment of premature ejaculation. BJU Int 2008; 102: 668–675.
McMahon CG . Treatment of premature ejaculation with sertraline hydrochloride: a single-blind placebo controlled crossover study. J Urol 1998; 159: 1935–1938.
McMahon CG, Touma K . Treatment of premature ejaculation with paroxetine hydrochloride. Int J Impot Res 1999; 11: 241–245; discussion 246.
McMahon CG, Touma K . Treatment of premature ejaculation with paroxetine hydrochloride as needed: 2 single-blind placebo controlled crossover studies. J Urol 1999; 161: 1826–1830.
Pryor JL, Althof SE, Steidle C, Rosen RC, Hellstrom WJ, Shabsigh R et al. Efficacy and tolerability of dapoxetine in treatment of premature ejaculation: an integrated analysis of two double-blind, randomised controlled trials. Lancet 2006; 368: 929–937.
Taber MT, Wright RN, Molski TF, Clarke WJ, Brassil PJ, Denhart DJ et al. Neurochemical, pharmacokinetic, and behavioral effects of the novel selective serotonin reuptake inhibitor BMS-505130. Pharmacol Biochem Behav 2005; 80: 521–528.
Mathias N, Moench P, Heran C, Wall D, Smith R . Alternate routes of administration of a selective serotonin reuptake inhibitor in rabbits and evaluation of different dosage forms. The APPS Journal 2006; 8 (S2): Abstract 1563.
VIVUS Inc.. VIVUS Reports Data from Premature Ejaculation Proof-of-Concept Trial. Business Wire. Press release, 2003 [cited 2008 August]; Available from: http://ir.vivus.com/phoenix.zhtml?c=79161&p=irol-newsArticle&ID=427244&highlight=.
Abn B, Kang KK, Lee YG, Choi SM, Kim DS, Yoo M . Abstract P-05-046: DA8031, a selective and potent serotonin transporter inhibitor, inhibits p-chloramphetamine-induced ejaculation in rats. J Sex Med 2008; 5 (Suppl 2): 59.
Celada P, Puig M, Amargos-Bosch M, Adell A, Artigas F . The therapeutic role of 5-HT1A and 5-HT2A receptors in depression. J Psychiatry Neurosci 2004; 29: 252–265.
Artigas F, Romero L, de Montigny C, Blier P . Acceleration of the effect of selected antidepressant drugs in major depression by 5-HT1A antagonists. Trends Neurosci 1996; 19: 378–383.
Ballesteros J, Callado LF . Effectiveness of pindolol plus serotonin uptake inhibitors in depression: a meta-analysis of early and late outcomes from randomised controlled trials. J Affect Disord 2004; 79: 137–147.
Looney C, Thor KB, Ricca D, Marson L . Differential effects of simultaneous or sequential administration of paroxetine and WAY-100,635 on ejaculatory behavior. Pharmacol Biochem Behav 2005; 82: 427–433.
de Jong TR, Pattij T, Veening JG, Dederen PJ, Waldinger MD, Cools AR et al. Citalopram combined with WAY 100635 inhibits ejaculation and ejaculation-related Fos immunoreactivity. Eur J Pharmacol 2005; 509: 49–59.
Williamson I, Turner L, Woods K, Wayman C, Van Der Graaf P . The 5-HT1A receptor antagonist robalzotan enhances SSRI-induced ejaculation delay in the rat. Br J Pharmacol 2003; 138 (Suppl 1): PO32.
Safarinejad MR . Once-daily high-dose pindolol for paroxetine-refractory premature ejaculation: a double-blind, placebo-controlled and randomized study. J Clin Psychopharmacol 2008; 28: 39–44.
Safarinejad MR, Hosseini SY . Safety and efficacy of tramadol in the treatment of premature ejaculation: a double-blind, placebo-controlled, fixed-dose, randomized study. J Clin Psychopharmacol 2006; 26: 27–31.
Salem EA, Wilson SK, Bissada NK, Delk JR, Hellstrom WJ, Cleves MA . Tramadol HCL has promise in on-demand use to treat premature ejaculation. J Sex Med 2008; 5: 188–193.
Mohammadi-Jazi A, Nori Mahdavi K, Salehi S . POS-01.88: study of efficacy and safety of oral tramadol in the treatment of premature ejaculation. Urology 2007; 70 (3 Suppl): 217.
DMI Biosciences Inc.. Clinical products: web page http://www.dmibio.com/index.php?option=com_content&task=view&id=26&Itemid=53. Access date: August 2008.
Palmer NR . Tramadol for premature ejaculation. J Sex Med 2008; [e-pub ahead of print] DOI: 10.1111/j.1743-6109.2008.00916.x.
McMahon CG, McMahon CN, Leow LJ, Winestock CG . Efficacy of type-5 phosphodiesterase inhibitors in the drug treatment of premature ejaculation: a systematic review. BJU Int 2006; 98: 259–272.
Hsieh JT, Liu SP, Hsieh CH, Cheng JT . An in vivo evaluation of the therapeutic potential of sympatholytic agents on premature ejaculation. BJU Int 1999; 84: 503–506.
Cavallini G . Alpha-1 blockade pharmacotherapy in primitive psychogenic premature ejaculation resistant to psychotherapy. Eur Urol 1995; 28: 126–130.
Basar MM, Yilmaz E, Ferhat M, Basar H, Batislam E . Terazosin in the treatment of premature ejaculation: a short-term follow-up. Int Urol Nephrol 2005; 37: 773–777.
Carmichael MS, Humbert R, Dixen J, Palmisano G, Greenleaf W, Davidson JM . Plasma oxytocin increases in the human sexual response. J Clin Endocrinol Metab 1987; 64: 27–31.
Arletti R, Bazzani C, Castelli M, Bertolini A . Oxytocin improves male copulatory performance in rats. Horm Behav 1985; 19: 14–20.
Arletti R, Benelli A, Bertolini A . Sexual behavior of aging male rats is stimulated by oxytocin. Eur J Pharmacol 1990; 179: 377–381.
Clement P, Peeters M, Bernabe J, Denys P, Alexandre L, Giuliano F . Brain oxytocin receptors mediate ejaculation elicited by 7-hydroxy-2-(di-N-propylamino) tetralin (7-OH-DPAT) in anaesthetized rats. Br J Pharmacol 2008; 154: 1150–1159.
Morales A, Barada J, Wyllie MG . A review of the current status of topical treatments for premature ejaculation. BJU Int 2007; 100: 493–501.
Berkovitch M, Keresteci AG, Koren G . Efficacy of prilocaine-lidocaine cream in the treatment of premature ejaculation. J Urol 1995; 154: 1360–1361.
Atikeler MK, Gecit I, Senol FA . Optimum usage of prilocaine-lidocaine cream in premature ejaculation. Andrologia 2002; 34: 356–359.
Busato W, Galindo CC . Topical anaesthetic use for treating premature ejaculation: a double-blind, randomized, placebo-controlled study. BJU Int 2004; 93: 1018–1021.
Henry R, Morales A, Wyllie MG . TEMPE: topical eutectic-like mixture for premature ejaculation. Expert Opin Drug Deliv 2008; 5: 251–261.
Dinsmore WW, Hackett G, Goldmeier D, Waldinger M, Dean J, Wright P et al. Topical eutectic mixture for premature ejaculation (TEMPE): a novel aerosol-delivery form of lidocaine-prilocaine for treating premature ejaculation. BJU Int 2007; 99: 369–375.
Gittleman MC, Mo J, Lu M . Synergistic effect of meatal application of dyclonine/alprostadil cream for the treatment of early ejaculation (EE) in a double-blind and crossover study. Proceedings of the 8th Congress of the European Society for Sexual Medicine, Copenhagen, Denmark. J Sex Med 2006; 3 (Suppl 3): 176.
Futura Medical. Product description: PET500, [cited 2008 August]; Available from: http://www.futuramedical.co.uk/content/products/pet_500.asp.
Xin ZC, Choi YD, Lee SH, Choi HK . Efficacy of a topical agent SS-cream in the treatment of premature ejaculation: preliminary clinical studies. Yonsei Med J 1997; 38: 91–95.
Fein RL . Intracavernous medication for treatment of premature ejaculation. Urology 1990; 35: 301–303.
Masters WH, Johnson VE . Human Sexual Inadequacy. Bantam, 1980: Toronto; London, 1970, x, 463pp.
Kaplan HS . The New Sex Therapy: Active Treatment of Sexual Dysfunctions. Brunner/Mazel: Levittown, PA; [Great Britain], 1974, xvi, 544pp.
American Psychological Association. Policy Statement on Evidence-Based Practice in Psychology 2005, [cited 2008 August]; Available from: http://www2.apa.org/practice/ebpstatement.pdf.
Perelman MA . A new combination treatment for premature ejaculation: a sex therapist's perspective. J Sex Med 2006; 3: 1004–1012.
Fischer Santos BO, deDues Vieira LA, Fischer R . Neurotomy: a new technique for the treatment of premature ejaculation. Int J Impotence Res 2001; 13 (Suppl 1): 11.
Hosseini SR, Khazaeli MH, Atharikia D . Role of postcircumcision mucosal cuff length in lifelong premature ejaculation: a pilot study. J Sex Med 2008; 5: 206–209.
Wise JME, Watson JP . A new treatment for premature ejaculation: case series for a desensitizing band. Sex Relationship Ther 2000; 15: 345–350.
Thomas CA, Tyagi S, Yoshimura N, Chancellor MB, Tyagi P . Effect of hyperforin-enriched extract on pro-ejaculatory effect of 8-hydroxy-2-(di-N-propylamino)tetralin in anesthetized rats. Urology 2007; 70: 813–816.
Song GH, Halmurat U, Geng JC, Feng LC, Yilihamujiang S, Ma C et al. Clinical study on the treatment of premature ejaculation by Uighur medicine gu-jing-mai-si-ha tablet. Chin J Integr Med 2007; 13: 185–189.
Ratnasooriya WD, Fernando TS . Effect of black tea brew of Camellia sinensis on sexual competence of male rats. J Ethnopharmacol 2008; 118: 373–377.
Author information
Authors and Affiliations
Corresponding author
Additional information
Conflict of interest
MG Wyllie is a Board Member of Plethora Solutions and JA Powell is a contractor to Plethora Solutions.
Rights and permissions
About this article
Cite this article
Powell, J., Wyllie, M. ‘Up and coming’ treatments for premature ejaculation: progress towards an approved therapy. Int J Impot Res 21, 107–115 (2009). https://doi.org/10.1038/ijir.2008.67
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ijir.2008.67