Abstract
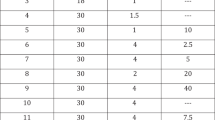
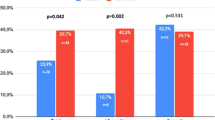
In two randomized, double-blind, placebo-controlled trials of 100 mg sildenafil citrate, men (N=601) with mild to moderate erectile dysfunction (ED) attempted intercourse 8 h (range, 7–9 h) postdose. The baseline to end-of-treatment improvement in the sildenafil groups vs placebo was greater (P<0.001) for the per-patient proportion (PPP) of ‘yes’ responses to the Sexual Encounter Profile question 3 (SEP3: successful intercourse (primary outcome)) (odds ratio (OR)=3.2 (trial 1), 7.6 (trial 2) and 5.6 (pooled data)); PPP of erection hardness score 4 (EHS 4, completely hard and fully rigid) (OR=6.2 (trial 1) and 10.9 (trial 2)); scores on the International Index of Erectile Function; and other EHS and SEP outcomes. Two to three times as many men were satisfied with sildenafil vs placebo treatment (Erectile Dysfunction Inventory of Treatment Satisfaction Index >50). Thus, responsiveness to 100 mg sildenafil may persist for 8 h postdose in men with mild to moderate ED.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 8 print issues and online access
$259.00 per year
only $32.38 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Feldman HA, Goldstein I, Hatzichristou DG, Krane RJ, McKinlay JB . Construction of a surrogate variable for impotence in the Massachusetts Male Aging Study. J Clin Epidemiol 1994; 47: 457–467.
Aytac IA, McKinley J, Krane RJ . The likely worldwide increase in erectile dysfunction between 1995 and 2025 and some possible policy consequences. BJU Int 1999; 84: 50–56.
Billups KL, Bank AJ, Padma-Nathan H, Katz S, Williams R . Erectile dysfunction is a marker for cardiovascular disease: results of the minority health institute expert advisory panel. J Sex Med 2005; 2: 40–50.
Althof SE . Quality of life and erectile dysfunction. Urology 2002; 59: 803–810.
Boolell M, Allen MJ, Ballard SA, Gepi-Attee S, Muirhead GJ, Naylor AM et al. Sildenafil: an orally active type 5 cyclic GMP-specific phosphodiesterase inhibitor for the treatment of penile erectile dysfunction. Int J Impot Res 1996; 8: 47–52.
Steers W, Guay AT, Leriche A, Gingell C, Hargreave TB, Wright PJ et al. Assessment of the efficacy and safety of Viagra (sildenafil citrate) in men with erectile dysfunction during long-term treatment. Int J Impot Res 2001; 13: 261–267.
Gingell C, Sultana S, Hodgson G, Wulff M, Gepi-Attee S . Duration of action of sildenafil citrate in men with erectile dysfunction. J Sex Med 2004; 1: 179–184.
Moncada I, Jara J, Subriá D, Castaño I, Hernández C . Efficacy of sildenafil citrate at 12 h after dosing: re-exploring the therapeutic window. Eur Urol 2004; 46: 357–361.
Muirhead GJ, Wilner K, Colburn W, Haug-Pihale G, Rouvieux B . The effects of age and renal and hepatic impairment on the pharmacokinetics of sildenafil citrate. Br J Clin Pharmacol 2002; 53: 21S–30S.
Gingell C, Buvat J, Jardin A, Olsson AM, Dinsmore WW, Maytom MC et al. Sildenafil citrate (VIAGRA), an oral treatment for erectile function: 1-year, open-label, extension studies. Multicentre Study Group. Int J Clin Pract Suppl 1999; 102: 30–31.
McCullough A, Steidle C, Klee B, Tseng L-J, Pace C . Randomized, double-blind, crossover trial of sildenafil in men with mild to moderate erectile dysfunction: efficacy at 8 and 12 h postdose. Urology 2008; 71: 686–692.
NIH Consensus Development Panel on Impotence. NIH Consensus Conference. Impotence. JAMA 1993; 270: 83–90.
Cappelleri JC, Rosen RC, Smith MD, Mishra A, Osterloh IH . Diagnostic evaluation of the erectile function domain of the International Index of Erectile Function. Urology 1999; 54: 346–351.
Lammers PI, Rubio-Aurioles E, Castell R, Castaneda J, Ponce De Leon R, Hurley D et al. Combination therapy for erectile dysfunction: a randomized, double blind, unblinded active-controlled, cross-over study of the pharmacodynamics and safety of combined oral formulations of apomorphine hydrochloride, phentolamine mesylate and papaverine hydrochloride in men with moderate to severe erectile dysfunction. Int J Impot Res 2002; 14: 54–59.
Mulhall JP, Goldstein I, Bushmakin AG, Cappelleri JC, Hvidsten K . Validation of the Erection Hardness Score (EHS). J Sex Med 2007; 4: 1626–1634.
Rosen RC, Riley A, Wagner G, Osterloh IH, Kirkpatrick J, Mishra A . The International Index of Erectile Function (IIEF): a multidimensional scale for assessment of erectile dysfunction. Urology 1997; 49: 822–830.
Althof SE, Corty EW, Levine SB, Levine F, Burnett AL, McVary K et al. EDITS: development of questionnaires for evaluating satisfaction with treatments for erectile dysfunction. Urology 1999; 53: 793–799.
SAS Institute Inc. SAS/STAT® User's Guide. Version 8. SAS Institute: Cary, NC, 1999.
Padma-Nathan H, Stecher VJ, Sweeney M, Orazem J, Tseng LJ, DeRiesthal H . Minimal time to successful intercourse after sildenafil citrate: results of a randomized, double-blind, placebo-controlled trial. Urology 2003; 62: 400–403.
LEVITRA®. Full Prescribing Information, Vardenafil. Bayer Pharmaceuticals Corporation: West Haven, CT, 2007.
CIALIS®. Full Prescribing Information, Tadalafil. Lilly ICOS, LLC: Indianapolis, IN, 2008.
Porst H, Sharlip ID, Hatzichristou D, Rubio-Aurioles E, Gittelman M, Stancil BN et al. Extended duration of efficacy of vardenafil when taken 8 h before intercourse: a randomized, double-blind, placebo-controlled study. Eur Urol 2006; 50: 1086–1094.
Porst H, Padma-Nathan H, Giuliano F, Anglin G, Varanese L, Rosen R . Efficacy of tadalafil for the treatment of erectile dysfunction at 24 and 36 h after dosing: a randomized controlled trial. Urology 2003; 62: 121–125.
Young JM, Feldman RA, Auerbach SM, Kaufman JM, Garcia CS, Shen W et al. Tadalafil improved erectile function at twenty-four and thirty-six hours after dosing in men with erectile dysfunction: US trial. J Androl 2005; 26: 310–318.
Nichols DJ, Muirhead GJ, Harness JA . Pharmacokinetics of sildenafil citrate after single oral doses in healthy male subjects: absolute bioavailability, food effects and dose proportionality. Br J Clin Pharmacol 2002; 53: 5S–12S.
Francis SH, Corbin JD . Molecular mechanisms and pharmacokinetics of phosphodiesterase-5 antagonists. Curr Urol Rep 2003; 4: 457–465.
Padma-Nathan H, Siegel R, Stecher V, DeRiesthal H . The association between minimum time to onset of action of sildenafil and minimum effective plasma sildenafil levels. J Androl 2005; (Suppl): 51.
Purvis K, Muirhead GJ, Harness JA . The effects of sildenafil on human sperm function in healthy volunteers. Br J Clin Pharmacol 2002; 53: 53S–60S.
Data on File. Pfizer Inc: New York, NY, 2007.
Acknowledgements
Study investigators included Connie Abarikwu, Stephen Auerbach, James Bailen, Martin Bastuba, David Bilhartz, Orpheus Bizzozero Jr, Joseph Camps Jr, Michael Collins, Lawrence Feldman, Robert Feldman, Sheldon Freedman, Evan Goldfischer, Gerard Henry, Adrian James, Jed Kaminetsky, Joel Kaufman, Edward Loizides, Andrew McCullough, James McMurray, Marcia Miller, Simon Mirelman, William Monnig, William Moseley Jr, Patrick Ogilvie, Terri Oskin, Juan Otheguy, Harin Padma-Nathan, Walter Pittman, Steven Rosenberg, Randolph Ross, Jose Sotolongo Jr, Christopher Steidle, Mark Swierzewski and David Talley. The studies were funded by Pfizer Inc. Editorial assistance was provided by Deborah M Campoli-Richards, BSPHA, RPh, and Nancy E Price, PhD, of Complete Healthcare Communications Inc., and was funded by Pfizer Inc.
Author information
Authors and Affiliations
Corresponding author
Additional information
Conflict of interest
Andrew McCullough has been a consultant, investigator, lecturer and/or Advisory Board member for Auxillium Pharmaceuticals, Bayer-GSK, Guilford Pharmaceuticals, Ion Channel, Johnson & Johnson, Lilly-ICOS, Pfizer and Schering Plough; Christopher P Steidle has been a lecturer for Astellas Pharma, Auxillium Pharmaceuticals Pfizer and Solvay Pharmaceuticals. Joel Kaufman has been a consultant, lecturer, trial investigator and/or on the Speakers’ Bureau for Amgen, Astellas Pharma, Coloplast, Indevus Pharmaceuticals, Lilly, Novartis Pharmaceuticals, Pfizer and Solvay Pharmaceuticals. Evan R Goldfischer has been a consultant, investigator and/or lecturer for Amgen, Astellis Pharma, Auxillium Pharmaceuticals, Bayer-GSK, Indevus Pharmaceuticals, Johnson & Johnson, Lilly, Merck & Co., Novartis Pharmaceuticals, Ortho McNeil Pharmaceutical, Pfizer, Sanofi-Aventis, Schering Plough and Schwartz. Brian Klee and Martin Carlsson are employees of Pfizer lnc.
Rights and permissions
About this article
Cite this article
McCullough, A., Steidle, C., Kaufman, J. et al. Sildenafil citrate efficacy 8 h postdose in men with mild to moderate erectile dysfunction. Int J Impot Res 20, 388–395 (2008). https://doi.org/10.1038/ijir.2008.21
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ijir.2008.21