Abstract
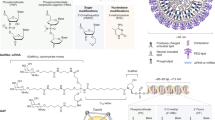
In the RNA interference process, the catalytic degradation of an endogenous mRNA results from the Watson-Crick complementary recognition by either a small silencing synthetic double-stranded ribonucleotide (siRNA) or by a small hairpin RNA (shRNA) produced in the cell by transcription from a DNA template. This interference process ideally results in an exquisitely specific mRNA suppression. The present review is dedicated to siRNAs. It describes the mechanism of RNA silencing and the main siRNA delivery techniques, with a focus on siRNA self-complexing to cationic lipids to form nanoparticles, which are called lipoplexes. The addition to lipoplexes of an anionic polymer leads to the ternary formulation APIRL (Anionic-Polymer-Interfering-RNA-Lipoplexes) with increased in vivo stability and biological efficacy. In terms of clinical development, the review focuses on therapeutic applications by intravenous delivery to the liver and inflammatory joints, and to localized siRNA delivery to the ocular sphere.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
Fire A, Xu S, Montgomery MK, Kostas SA, Driver SE, Mello CC . Potent and specific genetic interference by double-stranded RNA in Caenorhabditis elegans. Nature 1998; 391: 806–811.
Elbashir SM, Harborth J, Lendeckel W, Yalcin A, Weber K, Tuschl T . Duplexes of 21-nucleotide RNAs mediate RNA interference in cultured mammalian cells. Nature 2001; 411: 494–498.
Song E, Lee SK, Wang J, Ince N, Ouyang N, Min J et al. RNA interference targeting Fas protects mice from fulminant hepatitis. Nat Med 2003; 9: 347–351.
Aagaard L, Rossi JJ . RNAi therapeutics: principles, prospects and challenges. Adv Drug Deliver Rev 2007; 59: 75–86.
Morrissey DV, Lockridge JA, Shaw L, Blanchard K, Jensen K, Breen W et al. Potent and persistent in vivo anti-HBV activity of chemically modified siRNAs. Nat Biotech 2005; 23: 1002–1007.
Behlke MA . Chemical modification of siRNAs for in vivo use. Oligonucleotides 2008; 18: 305–319.
Engels JW . Gene silencing by chemically modified siRNAs. N Biotechnol 2013; 30: 302–307.
Marimani MD, Ely A, Buff MC, Bernhardt S, Engels JW, Arbuthnot P . Inhibition of hepatitis B virus replication in cultured cells and in vivo using 2'-O-guanidinopropyl modified siRNAs. Bioorg Med Chem 2013; 21: 6145–6155.
Parmar R, Willoughby JL, Liu J, Foster DJ, Brigham B, Theile CS et al. 5'-(E)-vinylphosphonate: a stable phosphate mimic can improve the RNAi activity of siRNA-GalNAc conjugates. Chembiochem 2016; 17: 985–989.
Essex S, Navarro G, Sabhachandani P, Chordia A, Trivedi M, Movassaghian S et al. Phospholipid-modified PEI-based nanocarriers for in vivo siRNA therapeutics against multidrug-resistant tumors. Gene Ther 2015; 22: 257–266.
Bouxsein NF, McAllister CS, Ewert KK, Samuel CE, Safinya CR . Structure and gene silencing activities of monovalent and pentavalent cationic lipid vectors complexed with siRNA. Biochemistry 2007; 46: 4785–4792.
Yang JP, Huang L . Overcoming the inhibitory effect of serum on lipofection by increasing the charge ratio of cationic liposome to DNA. Gene Ther 1997; 4: 950–960.
Schwartz B, Ivanov MA, Pitard B, Escriou V, Rangara R . Byk G at al. Synthetic DNA-compacting peptides derived from human sequence enhance cationic lipid-mediated gene transfer in vitro and in vivo. Gene Ther 1999; 6: 282–292.
Faneca H, Simoes S, Pedroso de Lima MC . Association of albumin or protamine to lipoplexes: enhancement of transfection and resistance to serum. J Gene Med 2004; 6: 681–692.
Escriou V, Carrière M, Bussone F, Wils P, Scherman D . Critical assessment of the nuclear import of plasmid during cationic lipid-mediated gene transfer. J Gene Med 2001; 3: 179–187.
Ewert KK, Zidovska A, Ahmad A, Bouxsein NF, Evans HM, McAllister CS et al. Cationic liposome-nucleic acid complexes for gene delivery and silencing: pathways and mechanisms for plasmid DNA and siRNA. Top Curr Chem 2010; 296: 191–226.
Rhinn H, Largeau C, Bigey P, Kuen RL, Richard M, Scherman D et al. How to make siRNA lipoplexes efficient? Add a DNA cargo. Biochim Biophys Acta 2009; 1790: 219–230.
Schlegel A, Largeau C, Bigey P, Bessodes M, Lebozec K, Scherman D et al. Anionic polymers for decreased toxicity and enhanced in vivo delivery of siRNA complexed with cationic liposomes. J Control Release 2011; 152: 393–401.
Li C, Wallace S . Polymer-drug conjugates: recent development in clinical oncology. Adv Drug Deliv Rev 2008; 60: 886–898.
Baron Esquivias G, Asteggiano R . Cardiac Management of Oncology Patients. Clinical Handbook for Cardio-Oncology: Springer International Publishing, Switzerland, 2015.
Schlegel A, Bigey P, Dhotel H, Scherman D, Escriou V . Reduced in vitro and in vivo toxicity of siRNA-lipoplexes with addition of polyglutamate. J Control Release 2013; 165: 1–8.
Hamoudi M, Henry E, Zerrouk N, Scherman D, Arnaud P, Deprez E et al. Enhancement of siRNA lipid-based vector stability and siRNA integrity in human serum with addition of anionic polymer adjuvant. J Drug Deliv Sci Technol 2015; 26: 1–9.
Kurreck J . Proof of RNA interference in humans after systemic delivery of siRNAs. Angew Chem Int Ed Engl 2010; 49: 6258–6259.
Sarisozen C, Salzano G, Torchilin VP . Lipid-based siRNA delivery dystems: challenges, promises and solutions along the long journey. Curr Pharm Biotechnol 2016; 17: 728–740.
Akinc A, Goldberg M, Qin J, Dorkin JR, Gamba-Vitalo C, Maier M et al. Development of lipidoid-siRNA formulations for systemic delivery to the liver. Mol Ther 2009; 17: 872–879.
Akinc A, Querbes W, De S, Qin J, Frank-Kamenetsky M, Jayaprakash KN et al. Targeted delivery of RNAi therapeutics with endogenous and exogenous ligand-based mechanisms. Mol Ther 2010; 18: 1357–1364.
Sato Y, Hatakeyama H, Hyodo M, Harashima H . Relationship between the physicochemical properties of lipid nanoparticles and the quality of siRNA delivery to liver cells. Mol Ther 2016; 24: 788–795.
Yamamoto N, Sato Y, Munakata T, Kakuni M, Tateno C, Sanada T et al. Novel pH-sensitive multifunctional envelope-type nanodevice for siRNA-based treatments for chronic HBV infection. J Hepatol 2016; 64: 547–555.
Khoury M, Louis-Plence P, Escriou V, Noel D, Largeau C, Cantos C et al. Efficient new cationic liposome formulation for systemic delivery of small interfering RNA silencing tumor necrosis factor alpha in experimental arthritis. Arthritis Rheum 2006; 54: 1867–1877.
Khoury M, Escriou V, Courties G, Galy A, Yao R, Largeau C et al. Efficient suppression of murine arthritis by combined anticytokine small interfering RNA lipoplexes. Arthritis Rheum 2008; 58: 2356–2367.
Rousseau J, Escriou V, Lamoureux F, Brion R, Chesneau J, Battaglia S et al. Formulated siRNAs targeting Rankl prevent osteolysis and enhance chemotherapeutic response in osteosarcoma models. J Bone Miner Res 2011; 26: 2452–2462.
Ozcan G, Ozpolat B, Coleman RL, Sood AK, Lopez-Berestein G . Preclinical and clinical development of siRNA-based therapeutics. Adv Drug Deliv Rev 2015; 87: 108–119.
Coelho T, Adams D, Silva A, Lozeron P, Hawkins PN, Mant T et al. Safety and efficacy of RNAi therapy for transthyretin amyloidosis. N Engl J Med 2013; 369: 819–829.
Guzman-Aranguez A, Loma P, Pintor J . Small-interfering RNAs (siRNAs) as a promising tool for ocular therapy. Br J Pharmacol 2013; 170: 730–747.
Kaiser PK, Symons RC, Shah SM, Quinlan EJ, Tabandeh H, Do DV et al. RNAi-based treatment for neovascular age-related macular degeneration by Sirna-027. Am J Ophthalmol 2010; 150: 33–39.
Nguyen QD, Schachar RA, Nduaka CI, Sperling M, Klamerus KJ, Chi-Burris K et al. Evaluation of the siRNA PF-04523655 versus ranibizumab for the treatment of neovascular age-related macular degeneration (MONET Study). Ophthalmology 2012; 119: 1867–1873.
Nguyen QD, Schachar RA, Nduaka CI, Sperling M, Basile AS, Klamerus KJ et al. Dose-ranging evaluation of intravitreal siRNA PF-04523655 for diabetic macular edema (the DEGAS study). Invest Ophthalmol Vis Sci 2012; 53: 7666–7674.
Moreno-Montañés J, Sádaba B, Ruz V, Gómez-Guiu A, Zarranz J, González MV et al. Phase I clinical trial of SYL040012, a small interfering RNA targeting β-adrenergic receptor 2, for lowering intraocular pressure. Mol Ther 2014; 22: 226–232.
Boudreau RL, Spengler RM, Davidson BL . Rational design of therapeutic siRNAs: minimizing off-targeting potential to improve the safety of RNAi therapy for Huntington's disease. Mol Ther 2011; 19: 2169–2177.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
DS, VE and PB are coauthors of the patent protecting the APIRL formulations: US 2012093915, Vectors including an anionic macromolecule and a cationic lipid for delivering small nucleic acids. The remaining author declares no conflict of interest.
Rights and permissions
About this article
Cite this article
Scherman, D., Rousseau, A., Bigey, P. et al. Genetic pharmacology: progresses in siRNA delivery and therapeutic applications. Gene Ther 24, 151–156 (2017). https://doi.org/10.1038/gt.2017.6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/gt.2017.6
This article is cited by
-
Redox-Responsive and Electrically Neutral PLGA Nanoparticles for siRNA Delivery in Human Cervical Carcinoma Cells
Journal of Pharmaceutical Innovation (2022)