Abstract
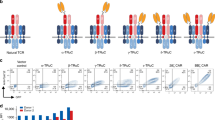
Adoptive cell therapy with chimeric antigen receptor (CAR)-modified T cells showed remarkable therapeutic efficacy in the treatment of leukaemia/lymphoma. However, the application to a variety of cancer entities is often constricted by the non-availability of a single chain antibody (scFv), which is usually the targeting domain in a CAR, while antibodies in the natural format are often available. To overcome the limitation, we designed a CAR that uses an antibody in its natural configuration for binding. Such CAR consists of two chains, the immunoglobulin light and heavy chain with their constant regions, whereby the heavy chain is anchored to the membrane and linked to an intracellular signalling domain for T-cell activation. The two chains form a stable heterodimer, a so-called dual chain CAR (dcCAR), and bind with high affinity and in a specific manner to their cognate antigen. By specific binding, the dcCAR activates engineered T cells for the release of pro-inflammatory cytokines and for target cell lysis. We provide evidence by three examples that the dcCAR format is universally applicable and thereby broadens the CAR cell therapy towards a larger variety of targets for which an scFv antibody is not available.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Lorentzen CL, Straten PT . CD19-chimeric antigen receptor T cells for treatment of chronic lymphocytic leukaemia and acute lymphoblastic leukaemia. Scand J Immunol 2015; 82: 307–319.
Porter DL, Hwang W, Frey NV, Lacey SF, Shaw PA, Loren AW et al. Chimeric antigen receptor T cells persist and induce sustained remissions in relapsed refractory chronic lymphocytic leukemia. Sci Transl Med 2015; 7: 303ra139.
Ewert S, Honegger A, Plückthun A . Structure-based improvement of the biophysical properties of immunoglobulin VH domains with a generalizable approach. Biochemistry 2003; 42: 1517–1528.
Ewert S, Huber T, Honegger A, Plückthun A . Biophysical properties of human antibody variable domains. J Mol Biol 2003; 325: 531–553.
Schaefer JV, Plückthun A . Transfer of engineered biophysical properties between different antibody formats and expression systems. Protein Eng Des Sel 2012; 25: 485–506.
Benedict CA, MacKrell AJ, Anderson WF . Determination of the binding affinity of an anti-CD34 single-chain antibody using a novel, flow cytometry based assay. J Immunol Methods 1997; 201: 223–231.
Lamers CHJ, Willemsen R, van Elzakker P, van Steenbergen-Langeveld S, Broertjes M, Oosterwijk-Wakka J et al. Immune responses to transgene and retroviral vector in patients treated with ex vivo-engineered T cells. Blood 2011; 117: 72–82.
Maus MV, Haas AR, Beatty GL, Albelda SM, Levine BL, Liu X et al. T cells expressing chimeric antigen receptors can cause anaphylaxis in humans. Cancer Immunol Res 2013; 1: 26–31.
Kershaw MH, Westwood JA, Parker LL, Wang G, Eshhar Z, Mavroukakis SA et al. A phase I study on adoptive immunotherapy using gene-modified T cells for ovarian cancer. Clin Cancer Res 2006; 12 (Pt 1): 6106–6115.
Gross G, Waks T, Eshhar Z . Expression of immunoglobulin-T-cell receptor chimeric molecules as functional receptors with antibody-type specificity. Proc Natl Acad Sci USA 1989; 86: 10024–10028.
Guest RD, Hawkins RE, Kirillova N, Cheadle EJ, Arnold J, O'Neill A et al. The role of extracellular spacer regions in the optimal design of chimeric immune receptors: evaluation of four different scFvs and antigens. J Immunother 2005; 28: 203–211.
Hombach A, Heuser C, Gerken M, Fischer B, Lewalter K, Diehl V et al. T cell activation by recombinant FcepsilonRI gamma-chain immune receptors: an extracellular spacer domain impairs antigen-dependent T cell activation but not antigen recognition. Gene Therapy 2000; 7: 1067–1075.
Muniappan A, Banapour B, Lebkowski J, Talib S . Ligand-mediated cytolysis of tumor cells: use of heregulin-zeta chimeras to redirect cytotoxic T lymphocytes. Cancer Gene Ther 2000; 7: 128–134.
Altenschmidt U, Kahl R, Moritz D, Schnierle BS, Gerstmayer B, Wels W et al. Cytolysis of tumor cells expressing the Neu/erbB-2, erbB-3, and erbB-4 receptors by genetically targeted naive T lymphocytes: receptor ligands as antigen-binding domains. Clin Cancer Res 1996; 2: 1001–1008.
Niederman TMJ, Ghogawala Z, Carter BS, Tompkins HS, Russell MM, Mulligan RC . Antitumor activity of cytotoxic T lymphocytes engineered to target vascular endothelial growth factor receptors. Proc Natl Acad Sci USA 2002; 99: 7009–7014.
Kahlon KS, Brown C, Cooper LJ, Raubitschek A, Forman SJ, Jensen MC . Specific recognition and killing of glioblastoma multiforme by interleukin 13-zetakine redirected cytolytic T cells. Cancer Res 2004; 64: 9160–9166.
Kong S, Sengupta S, Tyler B, Bais AJ, Ma Q, Doucette S et al. Suppression of human glioma xenografts with second-generation IL13R-specific chimeric antigen receptor-modified T cells. Clin Cancer Res 2012; 18: 5949–5960.
Pameijer CRJ, Navanjo A, Meechoovet B, Wagner JR, Aguilar B, Wright CL et al. Conversion of a tumor-binding peptide identified by phage display to a functional chimeric T cell antigen receptor. Cancer Gene Ther 2007; 14: 91–97.
Peh CA, Burrows SR, Barnden M, Khanna R, Cresswell P, Moss DJ et al. HLA-B27–restricted antigen presentation in the absence of tapasin reveals polymorphism in mechanisms of HLA class I peptide loading. Immunity 1998; 8: 531–542.
Bird LA, Peh CA, Kollnberger S, Elliott T, McMichael AJ, Bowness P . Lymphoblastoid cells express HLA-B27 homodimers both intracellularly and at the cell surface following endosomal recycling. Eur J Immunol 2003; 33: 748–759.
Hombach A, Wieczarkowiecz A, Marquardt T, Heuser C, Usai L, Pohl C et al. Tumor-specific T cell activation by recombinant immunoreceptors: CD3 signaling and CD28 costimulation are simultaneously required for efficient IL-2 secretion and can be integrated into one combined CD28/CD3 signaling receptor molecule. J Immunol 2001; 167: 6123–6131.
Hombach AA, Schildgen V, Heuser C, Finnern R, Gilham DE, Abken H . T cell activation by antibody-like immunoreceptors: the position of the binding epitope within the target molecule determines the efficiency of activation of redirected T cells. J Immunol 2007; 178: 4650–4657.
Cheadle EJ, Sheard V, Hombach AA, Chmielewski M, Riet T, Berrevoets C et al. Chimeric antigen receptors for T-cell based therapy. Methods Mol Biol 2012; 907: 645–666.
Acknowledgements
We thank Petra Hofmann and Nicole Riet for their technical assistance. The work was supported by a grant from the Deutsche Krebshilfe, Bonn and the Wilhelm Sander-Stiftung, München, Germany.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Rights and permissions
About this article
Cite this article
Faitschuk, E., Nagy, V., Hombach, A. et al. A dual chain chimeric antigen receptor (CAR) in the native antibody format for targeting immune cells towards cancer cells without the need of an scFv. Gene Ther 23, 718–726 (2016). https://doi.org/10.1038/gt.2016.48
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/gt.2016.48
This article is cited by
-
Chimäre Antigenrezeptoren (CAR) – universelle Werkzeuge in der zellulären Immuntherapie
Der Internist (2021)
-
Chimäre Antigenrezeptoren (CAR) – universelle Werkzeuge in der zellulären Immuntherapie
best practice onkologie (2021)
-
Chimäre Antigenrezeptoren (CAR) – universelle Werkzeuge in der zellulären Immuntherapie
Der Onkologe (2021)