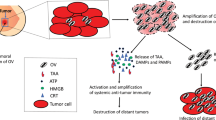
Abstract
Adeno-associated viral (AAV) vectors yield high potential for clinical gene therapy but, like for other vectors systems, they frequently do not sufficiently transduce the target tissue and their unspecific tropism prevents their application for multifocal diseases such as disseminated cancer. Targeted AAV vectors have been obtained from random AAV display peptide libraries but so far, all vector variants selected from AAV libraries upon systemic administration in vivo retained some collateral tropism, frequently the heart. Here we explored, if this impediment can be overcome by microRNA-regulated transgene cassettes as the combination of library-derived capsid targeting and micro-RNA control has not been evaluated so far. We used a tumor-targeted AAV capsid variant (ESGLSQS) selected from random AAV-display peptide libraries in vivo with remaining off-target tropism toward the heart and regulated targeted transgene expression in vivo by complementary target elements for heart-specific microRNA (miRT-1d). Although this vector still maintained its strong transduction capacity for tumor target tissue after intravenous injection, transgene expression in the heart was almost completely abrogated. This strong and completely tumor-specific transgene expression was used for therapeutic gene transfer in an aggressive multifocal, transgenic, polyoma middle T-induced, murine breast cancer model. A therapeutic suicide gene, delivered systemically by this dual-targeted AAV vector to multifocal breast cancer, significantly inhibited tumor growth after one single vector administration while avoiding side effects compared with untargeted vectors.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
Change history
08 October 2015
This article has been corrected since Advance Online Publication and a corrigendum is also printed in this issue
References
Brooks JD . Translational genomics: the challenge of developing cancer biomarkers. Genome Res 2012; 22: 183–187.
Seth P . Vector-mediated cancer gene therapy: an overview. Cancer Biol Ther 2005; 4: 512–517.
Kohlschutter J, Michelfelder S, Trepel M . Novel cytotoxic vectors based on adeno-associated virus. Toxins (Basel) 2011; 2: 2754–2768.
Michelfelder S, Trepel M . Adeno-associated viral vectors and their redirection to cell-type specific receptors. Adv Genet 2009; 67: 29–60.
Huttner NA, Girod A, Perabo L, Edbauer D, Kleinschmidt JA, Buning H et al. Genetic modifications of the adeno-associated virus type 2 capsid reduce the affinity and the neutralizing effects of human serum antibodies. Gene Therapy 2003; 10: 2139–2147.
Zhong L, Li B, Mah CS, Govindasamy L, Agbandje-McKenna M, Cooper M et al. Next generation of adeno-associated virus 2 vectors: point mutations in tyrosines lead to high-efficiency transduction at lower doses. Proc Natl Acad Sci USA 2008; 105: 7827–7832.
Maersch S, Huber A, Buning H, Hallek M, Perabo L . Optimization of stealth adeno-associated virus vectors by randomization of immunogenic epitopes. Virology 2009; 397: 167–175.
Girod A, Ried M, Wobus C, Lahm H, Leike K, Kleinschmidt J et al. Genetic capsid modifications allow efficient re-targeting of adeno-associated virus type 2. Nat Med 1999; 5: 1052–1056.
Wu P, Xiao W, Conlon T, Hughes J, Agbandje-McKenna M, Ferkol T et al. Mutational analysis of the adeno-associated virus type 2 (AAV2) capsid gene and construction of AAV2 vectors with altered tropism. J Virol 2000; 74: 8635–8647.
Loiler SA, Conlon TJ, Song S, Tang Q, Warrington KH, Agarwal A et al. Targeting recombinant adeno-associated virus vectors to enhance gene transfer to pancreatic islets and liver. Gene Therapy 2003; 10: 1551–1558.
Kern A, Schmidt K, Leder C, Muller OJ, Wobus CE, Bettinger K et al. Identification of a heparin-binding motif on adeno-associated virus type 2 capsids. J Virol 2003; 77: 11072–11081.
White SJ, Nicklin SA, Buning H, Brosnan MJ, Leike K, Papadakis ED et al. Targeted gene delivery to vascular tissue in vivo by tropism-modified adeno-associated virus vectors. Circulation 2004; 109: 513–519.
Work LM, Nicklin SA, Brain NJR, Dishart KL, Von Seggern DJ, Hallek M et al. Development of efficient viral vectors selective for vascular smooth muscle cells. Mol Ther 2004; 9: 198–208.
Work LM, Buning H, Hunt E, Nicklin SA, Denby L, Britton N et al. Vascular bed-targeted in vivo gene delivery using tropism-modified adeno-associated viruses. Mol Ther 2006; 13: 683–693.
White K, Buning H, Kritz A, Janicki H, McVey J, Perabo L et al. Engineering adeno-associated virus 2 vectors for targeted gene delivery to atherosclerotic lesions. Gene Therapy 2008; 15: 443–451.
Michelfelder S, Kohlschutter J, Skorupa A, Pfennings S, Muller O, Kleinschmidt JA et al. Successful expansion but not complete restriction of tropism of adeno-associated virus by in vivo biopanning of random virus display peptide libraries. PLoS One 2009; 4: e5122.
Pasqualini R, Ruoslahti E . Organ targeting in vivo using phage display peptide libraries. Nature 1996; 380: 364–366.
Arap W, Haedicke W, Bernasconi M, Kain R, Rajotte D, Krajewski S et al. Targeting the prostate for destruction through a vascular address. Proc Natl Acad Sci USA 2002; 99: 1527–1531.
Kolonin MG, Saha PK, Chan L, Pasqualini R, Arap W . Reversal of obesity by targeted ablation of adipose tissue. Nat Med 2004; 10: 625–632.
Munch RC, Janicki H, Volker I, Rasbach A, Hallek M, Buning H et al. Displaying high-affinity ligands on adeno-associated viral vectors enables tumor cell-specific and safe gene transfer. Mol Ther 2013; 21: 109–118.
Wickham TJ . Ligand-directed targeting of genes to the site of disease. Nat Med 2003; 9: 135–139.
Grimm D, Lee JS, Wang L, Desai T, Akache B, Storm TA et al. In vitro and in vivo gene therapy vector evolution via multispecies interbreeding and re-targeting of adeno-associated viruses. J Virol 2008; 82: 5887–5911.
Yang L, Jiang J, Drouin LM, Agbandje-McKenna M, Chen C, Qiao C et al. A myocardium tropic adeno-associated virus (AAV) evolved by DNA shuffling and in vivo selection. Proc Natl Acad Sci USA 2009; 106: 3946–3951.
Ying Y, Muller OJ, Goehringer C, Leuchs B, Trepel M, Katus HA et al. Heart-targeted adeno-associated viral vectors selected by in vivo biopanning of a random viral display peptide library. Gene Therapy 2010; 17: 980–990.
Michelfelder S, Varadi K, Raupp C, Hunger A, Korbelin J, Pahrmann C et al. Peptide ligands incorporated into the threefold spike capsid domain to re-direct gene transduction of AAV8 and AAV9 in vivo. PLoS One 2011; 6: e23101.
Brown BD, Cantore A, Annoni A, Sergi LS, Lombardo A, Della Valle P et al. A microRNA-regulated lentiviral vector mediates stable correction of hemophilia B mice. Blood 2007; 110: 4144–4152.
Cawood R, Chen HH, Carroll F, Bazan-Peregrino M, van Rooijen N, Seymour LW . Use of tissue-specific microRNA to control pathology of wild-type adenovirus without attenuation of its ability to kill cancer cells. PLoS Pathog 2009; 5: e1000440.
Qiao C, Yuan Z, Li J, He B, Zheng H, Mayer C et al. Liver-specific microRNA-122 target sequences incorporated in AAV vectors efficiently inhibits transgene expression in the liver. Gene Therapy 2010; 18: 403–410.
Geisler A, Jungmann A, Kurreck J, Poller W, Katus HA, Vetter R et al. microRNA122-regulated transgene expression increases specificity of cardiac gene transfer upon intravenous delivery of AAV9 vectors. Gene Therapy 2010; 18: 199–209.
Han C, Yu Z, Duan Z, Kan Q . Role of microRNA-1 in human cancer and its therapeutic potentials. BioMed Res Int 2014 2014: 428371.
Lin EY, Jones JG, Li P, Zhu L, Whitney KD, Muller WJ et al. Progression to malignancy in the polyoma middle T oncoprotein mouse breast cancer model provides a reliable model for human diseases. Am J Pathol 2003; 163: 2113–2126.
Guo K, Li J, Tang JP, Tan CP, Hong CW, Al-Aidaroos AQ et al. Targeting intracellular oncoproteins with antibody therapy or vaccination. Sci Transl Med 2011; 3: 99ra85.
Fend L, Accart N, Kintz J, Cochin S, Reymann C, Le Pogam F et al. Therapeutic effects of anti-CD115 monoclonal antibody in mouse cancer models through dual inhibition of tumor-associated macrophages and osteoclasts. PLoS One 2013; 8: e73310.
Hallett MA, Teng B, Hasegawa H, Schwab LP, Seagroves TN, Pourmotabbed T . Anti-matrix metalloproteinase-9 DNAzyme decreases tumor growth in the MMTV-PyMT mouse model of breast cancer. Breast Cancer Res 2013; 15: R12.
Vandenberghe LH, Wang L, Somanathan S, Zhi Y, Figueredo J, Calcedo R et al. Heparin binding directs activation of T cells against adeno-associated virus serotype 2 capsid. Nat Med 2006; 12: 967–971.
Moskalenko M, Chen L, van Roey M, Donahue BA, Snyder RO, McArthur JG et al. Epitope mapping of human anti-adeno-associated virus type 2 neutralizing antibodies: implications for gene therapy and virus structure. J Virol 2000; 74: 1761–1766.
Basner-Tschakarjan E, Mingozzi F . Cell-mediated immunity to AAV vectors, evolving concepts and potential solutions. Front Immunol 2014; 5: 350.
Jiang H, Couto LB, Patarroyo-White S, Liu T, Nagy D, Vargas JA et al. Effects of transient immunosuppression on adenoassociated, virus-mediated, liver-directed gene transfer in rhesus macaques and implications for human gene therapy. Blood 2006; 108: 3321–3328.
Wang Z, Storb R, Halbert CL, Banks GB, Butts TM, Finn EE et al. Successful regional delivery and long-term expression of a dystrophin gene in canine muscular dystrophy: a preclinical model for human therapies. Mol Ther 2012; 20: 1501–1507.
Hui DJ, Basner-Tschakarjan E, Chen Y, Davidson RJ, Buchlis G, Yazicioglu M et al. Modulation of CD8+ T cell responses to AAV vectors with IgG-derived MHC class II epitopes. Mol Ther 2013; 21: 1727–1737.
Jensen MM, Jorgensen JT, Binderup T, Kjaer A . Tumor volume in subcutaneous mouse xenografts measured by microCT is more accurate and reproducible than determined by 18 F-FDG-microPET or external caliper. BMC Med Imaging 2008; 8: 16.
Lagos-Quintana M, Rauhut R, Yalcin A, Meyer J, Lendeckel W, Tuschl T . Identification of tissue-specific microRNAs from mouse. Curr Biol 2002; 12: 735–739.
Hajitou A, Trepel M, Lilley CE, Soghomonyan S, Alauddin MM, Marini FC 3rd et al. A hybrid vector for ligand-directed tumor targeting and molecular imaging. Cell 2006; 125: 385–398.
Xiao X, Li J, Samulski RJ . Production of high-titer recombinant adeno-associated virus vectors in the absence of helper adenovirus. J Virol 1998; 72: 2224–2232.
Zolotukhin S, Byrne BJ, Mason E, Zolotukhin I, Potter M, Chesnut K et al. Recombinant adeno-associated virus purification using novel methods improves infectious titer and yield. Gene Therapy 1999; 6: 973–985.
Rohr UP, Heyd F, Neukirchen J, Wulf MA, Queitsch I, Kroener-Lux G et al. Quantitative real-time PCR for titration of infectious recombinant AAV-2 particles. J Virol Methods 2005; 127: 40–45.
Acknowledgements
We thank Dr Tomas Streichert, Department of clinical chemistry and the Core Facility for OIVI at the University Medical Center Hamburg Eppendorf for technical support and we thank the animal facility of the University Medical Center Hamburg-Eppendorf for excellent animal care. This work was supported by the German Research Foundation (DFG, grant numbers TR448/5-3 to MT, Mu1654/3-2 to JAK and 516/8-2 to JAK), the Deutsche Krebshilfe (grant number 110902 to MT) and the Margarethe Clemens Foundation (endowed professorship to MT).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Rights and permissions
About this article
Cite this article
Trepel, M., Körbelin, J., Spies, E. et al. Treatment of multifocal breast cancer by systemic delivery of dual-targeted adeno-associated viral vectors. Gene Ther 22, 840–847 (2015). https://doi.org/10.1038/gt.2015.52
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/gt.2015.52