Abstract
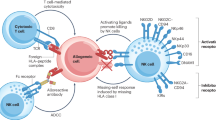
Hepatitis C virus (HCV)-induced, end-stage liver disease is a major indication for liver transplantation, but systematic graft reinfection accelerates liver disease recurrence. Transplantation recipients may be ineligible for direct-acting antivirals, owing to toxicity, resistance or advanced liver disease. Adoptive immunotherapy with liver graft-derived, ex vivo-activated lymphocytes was previously shown to prevent HCV-induced graft reinfections. Alternatively, the applicability and therapeutic efficacy of adoptive immunotherapy may be enhanced by ‘ready for use’ suicide gene-modified lymphocytes from healthy blood donors; moreover, conditional, prodrug-induced cell suicide may prevent potential side effects. Here, we demonstrate that allogeneic suicide gene-modified lymphocytes (SGMLs) could potently, dose- and time-dependently, inhibit viral replication. The effect occurs at effector:target cell ratios that exhibits no concomitant cytotoxicity toward virus-infected target cells. The effect, mediated mostly by CD56+ lymphocytes, is interleukin-2-dependent, IFN-γ-mediated and, importantly, resistant to calcineurin inhibitors. Thus, post-transplant immunosuppression may not interfere with this adoptive cell immunotherapy approach. Furthermore, these cells are indeed amenable to conditional cell suicide; in particular, the inducible caspase 9 suicide gene is superior to the herpes simplex virus thymidine kinase suicide gene. Our data provide in vitro proof-of-concept that allogeneic, third-party, SGMLs may prevent HCV-induced liver graft reinfection.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Global surveillance and control of hepatitis C. Report of a WHO Consultation organized in collaboration with the Viral Hepatitis Prevention Board, Antwerp, Belgium. J Viral Hepatitis 1999; 6: 35–47.
Skagen C, Lucey M, Said A . Liver transplantation: an update 2009. Curr Opin Gastroenterol 2009; 25: 202–208.
Gane EJ . The natural history of recurrent hepatitis C and what influences this. Liver Transpl 2008; 14: S36–S44.
Tai AW, Chung RT . Treatment failure in hepatitis C: mechanisms of non-response. J Hepatol 2009; 50: 412–420.
Curry MP, Forns X, Chung RT, Terrault N, Brown RS, Fenkel JM et al. Pretransplant sofosbuvir and ribavirin to prevent recurrence of HCV infection after liver transplantation. Hepatology 2013; 58: 314A–315A.
Forns X, Fontana RJ, Moonka D, McHutchison JG, Symonds WT, Denning JM et al. Initial evaluation of the sofosbuvir compassionate use program for patients with severe recurrent HCV following liver transplantation. Hepatology 2013; 58: 732A–733A.
Charlton M . Telaprevir, boceprevir, cytochrome P450 and immunosuppressive agents—a potentially lethal cocktail. Hepatology 2011; 54: 3–5.
Ohira M, Ishiyama K, Tanaka Y, Doskali M, Igarashi Y, Tashiro H et al. Adoptive immunotherapy with liver allograft-derived lymphocytes induces anti-HCV activity after liver transplantation in humans and humanized mice. J Clin Invest 2009; 119: 3226–3235.
Bonini C, Ferrari G, Verzeletti S, Servida P, Zappone E, Ruggieri L et al. HSV-TK gene transfer into donor lymphocytes for control of allogeneic graft-versus-leukemia. Science 1997; 276: 1719–1724.
Ciceri F, Bonini C, Stanghellini MTL, Bondanza A, Traversari C, Salomoni M et al. Infusion of suicide-gene-engineered donor lymphocytes after family haploidentical haemopoietic stem-cell transplantation for leukaemia (the TK007 trial): a non-randomised phase I-II study. Lancet Oncol 2009; 10: 489–500.
Zhou X, Di Stasi A, Tey SK, Krance RA, Martinez C, Leung KS et al. Long-term outcome after haploidentical stem cell transplant and infusion of T cells expressing the inducible caspase 9 safety transgene. Blood 2014; 123: 3895–3905.
Tiberghien P, Ferrand C, Lioure B, Milpied N, Angonin R, Deconinck E et al. Administration of herpes simplex-thymidine kinase-expressing donor T cells with a T-cell-depleted allogeneic marrow graft. Blood 2001; 97: 63–72.
Mercier-Letondal P, Deschamps M, Sauce D, Certoux JM, Milpied N, Lioure B et al. Early immune response against retrovirally transduced herpes simplex virus thymidine kinase-expressing gene-modified T cells coinfused with a T cell-depleted marrow graft: an altered immune response? Hum Gene Ther 2008; 19: 937–950.
Deschamps M, Mercier-Lethondal P, Certoux JM, Henry C, Lioure B, Pagneux C et al. Deletions within the HSV-tk transgene in long-lasting circulating gene-modified T cells infused with a hematopoietic graft. Blood 2007; 110: 3842–3852.
Mailly L, Leboeuf C, Tiberghien P, Baumert T, Robinet E . Genetically engineered T-cells expressing a ganciclovir-sensitive HSV-tk suicide gene for the prevention of GvHD. Curr Opin Investig Drugs 2010; 11: 559–570.
Sauce D, Tonnelier N, Duperrier A, Petracca B, de Carvalho Bittencourt M, Saadi M et al. Influence of ex vivo expansion and retrovirus-mediated gene transfer on primary T lymphocyte phenotype and functions. J Hematother Stem Cell Res 2002; 11: 929–940.
Mercier-Letondal P, Montcuquet N, Sauce D, Certoux JM, Jeanningros S, Ferrand C et al. Alloreactivity of ex vivo-expanded T cells is correlated with expansion and CD4/CD8 ratio. Cytotherapy 2008; 10: 275–288.
Contassot E, Robinet E, Angonin R, Laithier V, Bittencourt M, Pavy JJ et al. Differential effects of cyclosporin A on the alloreactivity of fresh and ex vivo-expanded T lymphocytes. Bone Marrow Transplant 1998; 22: 1097–1102.
El-Farrash MA, Aly HH, Watashi K, Hijikata M, Egawa H, Shimotohno K . In vitro infection of immortalized primary hepatocytes by HCV genotype 4a and inhibition of virus replication by cyclosporin. Microbiol Immunol 2007; 51: 127–133.
Marin V, Cribioli E, Philip B, Tettamanti S, Pizzitola I, Biondi A et al. Comparison of different suicide-gene strategies for the safety improvement of genetically manipulated T cells. Hum Gene Ther Methods 2012; 23: 376–386.
Doskali M, Tanaka Y, Ohira M, Ishiyama K, Tashiro H, Chayama K et al. Possibility of adoptive immunotherapy with peripheral blood-derived CD3(−)CD56+ and CD3+CD56+ cells for inducing antihepatocellular carcinoma and antihepatitis C virus activity. J Immunother 2011; 34: 129–138.
Leboeuf C, Mailly L, Wu T, Bour G, Durand S, Brignon N et al. In vivo proof of concept of adoptive immunotherapy for hepatocellular carcinoma using allogeneic suicide gene-modified killer cells. Mol Ther 2014; 22: 634–644.
Henrich TJ, Hu Z, Li JZ, Sciaranghella G, Busch MP, Keating SM et al. Long-term reduction in peripheral blood HIV type 1 reservoirs following reduced-intensity conditioning allogeneic stem cell transplantation. J Infect Dis 2013; 207: 1694–1702.
Cillo AR, Krishnan A, Mitsuyasu RT, McMahon DK, Li S, Rossi JJ et al. Plasma viremia and cellular HIV-1 DNA persist despite autologous hematopoietic stem cell transplantation for HIV-related lymphoma. J Acquir Immune Defic Syndr 2013; 63: 438–441.
Di Stasi A, Tey SK, Dotti G, Fujita Y, Kennedy-Nasser A, Martinez C et al. Inducible apoptosis as a safety switch for adoptive cell therapy. N Eng J Med 2011; 365: 1673–1683.
Mercer DF, Schiller DE, Elliott JF, Douglas DN, Hao C, Rinfret A et al. Hepatitis C virus replication in mice with chimeric human livers. Nat Med 2001; 7: 927–933.
Fehse B, Kustikova OS, Li Z, Wahlers A, Bohn W, Beyer WR et al. A novel 'sort-suicide' fusion gene vector for T cell manipulation. Gene Ther 2002; 9: 1633–1638.
Wakita T, Pietschmann T, Kato T, Date T, Miyamoto M, Zhao Z et al. Production of infectious hepatitis C virus in tissue culture from a cloned viral genome. Nat Med 2005; 11: 791–796.
Lindenbach BD, Evans MJ, Syder AJ, Wolk B, Tellinghuisen TL, Liu CC et al. Complete replication of hepatitis C virus in cell culture. Science 2005; 309: 623–626.
Lindenbach BD, Meuleman P, Ploss A, Vanwolleghem T, Syder AJ, McKeating JA et al. Cell culture-grown hepatitis C virus is infectious in vivo and can be recultured in vitro. Proc Natl Acad Sci USA 2006; 103: 3805–3809.
Pietschmann T, Kaul A, Koutsoudakis G, Shavinskaya A, Kallis S, Steinmann E et al. Construction and characterization of infectious intragenotypic and intergenotypic hepatitis C virus chimeras. Proc Natl Acad Sci USA 2006; 103: 7408–7413.
Zhong J, Gastaminza P, Cheng G, Kapadia S, Kato T, Burton DR et al. Robust hepatitis C virus infection in vitro. Proc Natl Acad Sci USA 2005; 102: 9294–9299.
Acknowledgements
This study was supported by Inserm, the Fondation pour la Recherche Médicale (Comité Alsace), the Ligue Nationale Contre le Cancer (Conférence de Coordination Inter-Régionale Grand-Est, grant # 1FI10005LBKD), the Association pour la recherche sur le Cancer (ARC, grant # SFI20111203529), the Société d’Accélération de Transfert de Technologie (SATT) Conectus Alsace, the Agence Nationale pour la Recherche (ANR, LabEx program) and EU Interreg-IV Program Hepato-Regio-Net. Eric Robinet was supported by the Agence Nationale pour la Recherche sur le SIDA et les Hépatites Virales (ANRS, grant # 2008 059 ULP). Céline Leboeuf received fellowships from the Fondation Transplantation and the Association pour la Recherche sur le Cancer (ARC, grant # DOC20110603384); and Tao Wu received a fellowship from the Alsace Region. A European Patent application (n° EP12305259.9) related to this study, entitled ‘Method for preventing or treating HCV infection by administration of suicide gene-modified lymphocytes’, was submitted on the 2 March 2012 by the University of Strasbourg.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Supplementary Information accompanies this paper on Gene Therapy website
Supplementary information
Rights and permissions
About this article
Cite this article
Leboeuf, C., Roser-Schilder, J., Lambotin, M. et al. Prevention of hepatitis C virus infection by adoptive allogeneic immunotherapy using suicide gene-modified lymphocytes: an in vitro proof-of-concept. Gene Ther 22, 172–180 (2015). https://doi.org/10.1038/gt.2014.99
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/gt.2014.99