Abstract
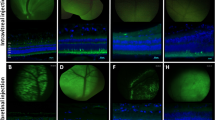
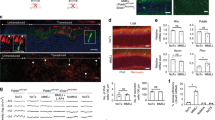
Gene therapy with adeno-associated viral (AAV) vectors is limited by AAV cargo capacity that prevents their application to the inherited retinal diseases (IRDs), such as Stargardt disease (STGD) or Usher syndrome type IB (USH1B), which are due to mutations in genes larger than 5 kb. Trans-splicing or hybrid dual AAV vectors have been successfully exploited to reconstitute large gene expression in the mouse retina. Here, we tested them in the large cone-enriched pig retina that closely mimics the human retina. We found that dual AAV trans-splicing and hybrid vectors transduce pig photoreceptors, the major cell targets for treatment of IRDs, to levels that were about two- to threefold lower than those obtained with a single AAV vector of normal size. This efficiency is significantly higher than that in mice, and is potentially due to the high levels of dual AAV co-transduction we observe in pigs. We also show that subretinal delivery in pigs of dual AAV trans-splicing and hybrid vectors successfully reconstitute, albeit at variable levels, the expression of the large genes ABCA4 and MYO7A mutated in STGD and USH1B, respectively. Our data support the potential of dual AAV vectors for large gene reconstitution in the cone-enriched pig retina that is a relevant preclinical model.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Sohocki MM, Daiger SP, Bowne SJ, Rodriquez JA, Northrup H, Heckenlively JR et al. Prevalence of mutations causing retinitis pigmentosa and other inherited retinopathies. Hum Mutat 2001; 17: 42–51.
Dryja T . Retinitis pigmentosa and stationary night blindness. In: Scriver C, Beaudet A, Sly W, Valle D (eds) The Online Metabolic & Molecular Bases of Inherited Diseases. McGraw-Hill: New York, 2001, pp 5903–5933.
Berger W, Kloeckener-Gruissem B, Neidhardt J . The molecular basis of human retinal and vitreoretinal diseases. Prog Retin Eye Res 2010; 29: 335–375.
Bainbridge JW, Smith AJ, Barker SS, Robbie S, Henderson R, Balaggan K et al. Effect of gene therapy on visual function in Leber's congenital amaurosis. N Engl J Med 2008; 358: 2231–2239.
Cideciyan AV, Hauswirth WW, Aleman TS, Kaushal S, Schwartz SB, Boye SL et al. Vision 1 year after gene therapy for Leber’s congenital amaurosis. N Engl J Med 2009; 361: 725–727.
Maguire AM, Simonelli F, Pierce EA, Pugh EN Jr, Mingozzi F, Bennicelli J et al. Safety and efficacy of gene transfer for Leber’s congenital amaurosis. N Engl J Med 2008; 358: 2240–2248.
Maguire AM, High KA, Auricchio A, Wright JF, Pierce EA, Testa F et al. Age-dependent effects of RPE65 gene therapy for Leber’s congenital amaurosis: a phase 1 dose-escalation trial. Lancet 2009; 374: 1597–1605.
Simonelli F, Maguire AM, Testa F, Pierce EA, Mingozzi F, Bennicelli JL et al. Gene therapy for Leber's congenital amaurosis is safe and effective through 1.5 years after vector administration. Mol Ther 2010; 18: 643–650.
Testa F, Maguire AM, Rossi S, Pierce EA, Melillo P, Marshall K et al. Three-year follow-up after unilateral subretinal delivery of adeno-associated virus in patients with Leber congenital amaurosis type 2. Ophthalmology 2013; 120: 1283–1291.
Allocca M, Mussolino C, Garcia-Hoyos M, Sanges D, Iodice C, Petrillo M et al. Novel adeno-associated virus serotypes efficiently transduce murine photoreceptors. J Virol 2007; 81: 11372–11380.
Mussolino C, della Corte M, Rossi S, Viola F, Di Vicino U, Marrocco E et al. AAV-mediated photoreceptor transduction of the pig cone-enriched retina. Gene Therapy 2011; 18: 637–645.
Vandenberghe LH, Bell P, Maguire AM, Cearley CN, Xiao R, Calcedo R et al. Dosage thresholds for AAV2 and AAV8 photoreceptor gene therapy in monkey. Sci Transl Med 2011; 3: 88ra54.
Auricchio A . Fighting blindness with adeno-associated virus serotype 8. Hum Gene Ther 2011; 22: 1169–1170.
Natkunarajah M, Trittibach P, McIntosh J, Duran Y, Barker SE, Smith AJ et al. Assessment of ocular transduction using single-stranded and self-complementary recombinant adeno-associated virus serotype 2/8. Gene Therapy 2008; 15: 463–467.
Boye SE, Alexander JJ, Boye SL, Witherspoon CD, Sandefer KJ, Conlon TJ et al. The human rhodopsin kinase promoter in an AAV5 vector confers rod- and cone-specific expression in the primate retina. Hum Gene Ther 2012; 23: 1101–1115.
Vandenberghe LH, Bell P, Maguire AM, Xiao R, Hopkins TB, Grant R et al. AAV9 targets cone photoreceptors in the nonhuman primate retina. PLoS One 2013; 8: e53463.
Hermonat PL, Quirk JG, Bishop BM, Han L . The packaging capacity of adeno-associated virus (AAV) and the potential for wild-type-plus AAV gene therapy vectors. FEBS Lett 1997; 407: 78–84.
Dong B, Nakai H, Xiao W . Characterization of genome integrity for oversized recombinant AAV vector. Mol Ther 2010; 18: 87–92.
Lai Y, Yue Y, Duan D . Evidence for the failure of adeno-associated virus serotype 5 to package a viral genome>or=8.2 kb. Mol Ther 2010; 18: 75–79.
Wu Z, Yang H, Colosi P . Effect of genome size on AAV vector packaging. Mol Ther 2010; 18: 80–86.
Allikmets R . A photoreceptor cell-specific ATP-binding transporter gene (ABCR) is mutated in recessive Stargardt macular dystrophy. Nat Genet 1997; 17: 122.
Millan JM, Aller E, Jaijo T, Blanco-Kelly F, Gimenez-Pardo A, Ayuso C . An update on the genetics of usher syndrome. J Ophthalmol 2011; 2011: 417217.
Hasson T, Heintzelman MB, Santos-Sacchi J, Corey DP, Mooseker MS . Expression in cochlea and retina of myosin VIIa, the gene product defective in Usher syndrome type 1B. Proc Natl Acad Sci USA 1995; 92: 9815–9819.
Liu X, Vansant G, Udovichenko IP, Wolfrum U, Williams DS . Myosin VIIa, the product of the Usher 1B syndrome gene, is concentrated in the connecting cilia of photoreceptor cells. Cell Motil Cytoskeleton 1997; 37: 240–252.
Gibbs D, Diemer T, Khanobdee K, Hu J, Bok D, Williams DS . Function of MYO7A in the human RPE and the validity of shaker1 mice as a model for Usher syndrome 1B. Invest Ophthalmol Vis Sci 2010; 51: 1130–1135.
Sahly I, Dufour E, Schietroma C, Michel V, Bahloul A, Perfettini I et al. Localization of Usher 1 proteins to the photoreceptor calyceal processes, which are absent from mice. J Cell Biol 2012; 199: 381–399.
Grieger JC, Samulski RJ . Packaging capacity of adeno-associated virus serotypes: impact of larger genomes on infectivity and postentry steps. J Virol 2005; 79: 9933–9944.
Wu J, Zhao W, Zhong L, Han Z, Li B, Ma W et al. Self-complementary recombinant adeno-associated viral vectors: packaging capacity and the role of rep proteins in vector purity. Hum Gene Ther 2007; 18: 171–182.
Allocca M, Doria M, Petrillo M, Colella P, Garcia-Hoyos M, Gibbs D et al. Serotype-dependent packaging of large genes in adeno-associated viral vectors results in effective gene delivery in mice. J Clin Invest 2008; 118: 1955–1964.
McIntosh J, Lenting PJ, Rosales C, Lee D, Rabbanian S, Raj D et al. Therapeutic levels of FVIII following a single peripheral vein administration of rAAV vector encoding a novel human factor VIII variant. Blood 2013; 121: 3335–3344.
Yan Z, Zhang Y, Duan D, Engelhardt JF . Trans-splicing vectors expand the utility of adeno-associated virus for gene therapy. Proc Natl Acad Sci USA 2000; 97: 6716–6721.
Duan D, Yue Y, Engelhardt JF . Expanding AAV packaging capacity with trans-splicing or overlapping vectors: a quantitative comparison. Mol Ther 2001; 4: 383–391.
Ghosh A, Duan D . Expanding adeno-associated viral vector capacity: a tale of two vectors. Biotechnol Genet Eng Rev 2007; 24: 165–177.
Ghosh A, Yue Y, Lai Y, Duan D . A hybrid vector system expands adeno-associated viral vector packaging capacity in a transgene-independent manner. Mol Ther 2008; 16: 124–130.
Hirsch ML, Li C, Bellon I, Yin C, Chavala S, Pryadkina M et al. Oversized AAV transductifon is mediated via a DNA-PKcs-independent, Rad51C-dependent repair pathway. Mol Ther 2013; 21: 2205–2216.
Monahan PE, Lothrop CD, Sun J, Hirsch ML, Kafri T, Kantor B et al. Proteasome inhibitors enhance gene delivery by AAV virus vectors expressing large genomes in hemophilia mouse and dog models: a strategy for broad clinical application. Mol Ther 2010; 18: 1907–1916.
Grose WE, Clark KR, Griffin D, Malik V, Shontz KM, Montgomery CL et al. Homologous recombination mediates functional recovery of dysferlin deficiency following AAV5 gene transfer. PLoS One 2012; 7: e39233.
Lopes VS, Boye SE, Louie CM, Boye S, Dyka F, Chiodo V et al. Retinal gene therapy with a large MYO7A cDNA using adeno-associated virus. Gene Therapy 2013; 20: 824–833.
Wang Y, Ling C, Song L, Wang L, Aslanidi GV, Tan M et al. Limitations of encapsidation of recombinant self-complementary adeno-associated viral genomes in different serotype capsids and their quantitation. Hum Gene Ther Methods 2012; 23: 225–233.
Colella P, Sommella A, Marrocco E, Di Vicino U, Polishchuk E, Garrido MG et al. Myosin7a deficiency results in reduced retinal activity which is improved by gene therapy. PLoS One 2013; 8: e72027.
Reich SJ, Auricchio A, Hildinger M, Glover E, Maguire AM, Wilson JM et al. Efficient trans-splicing in the retina expands the utility of adeno-associated virus as a vector for gene therapy. Hum Gene Ther 2003; 14: 37–44.
Duan D, Sharma P, Yang J, Yue Y, Dudus L, Zhang Y et al. Circular intermediates of recombinant adeno-associated virus have defined structural characteristics responsible for long-term episomal persistence in muscle tissue. J Virol 1998; 72: 8568–8577.
Trapani I, Colella P, Sommella A, Iodice C, Cesi G, De Simone S et al. Effective delivery of large genes to the retina by dual AAV vectors. EMBO Mol Med 2014; 6: 194–211.
Choi VW, Samulski RJ, McCarty DM . Effects of adeno-associated virus DNA hairpin structure on recombination. J Virol 2005; 79: 6801–6807.
Choi VW, McCarty DM, Samulski RJ . Host cell DNA repair pathways in adeno-associated viral genome processing. J Virol 2006; 80: 10346–10356.
Hendrickson A, Hicks D . Distribution and density of medium- and short-wavelength selective cones in the domestic pig retina. Exp Eye Res 2002; 74: 435–444.
Karali M, Manfredi A, Puppo A, Marrocco E, Gargiulo A, Allocca M et al. MicroRNA-restricted transgene expression in the retina. PLoS One 2011; 6: e22166.
Manfredi A, Marrocco E, Puppo A, Cesi G, Sommella A, Della Corte M et al. Combined rod and cone transduction by adeno-associated virus 2/8. Hum Gene Ther 2013; 2: 982–992.
Testa F, Surace EM, Rossi S, Marrocco E, Gargiulo A, Di Iorio V et al. Evaluation of Italian patients with leber congenital amaurosis due to AIPL1 mutations highlights the potential applicability of gene therapy. Invest Ophthalmol Vis Sci 2011; 52: 5618–5624.
Puppo A, Bello A, Manfredi A, Cesi G, Marrocco E, Della Corte M et al. Recombinant vectors based on porcine adeno-associated viral serotypes transduce the murine and pig retina. PLoS One 2013; 8: e59025.
Pang JJ, Dai X, Boye SE, Barone I, Boye SL, Mao S et al. Long-term retinal function and structure rescue using capsid mutant AAV8 vector in the rd10 mouse, a model of recessive retinitis pigmentosa. Mol Ther 2011; 19: 234–242.
Tan MH, Smith AJ, Pawlyk B, Xu X, Liu X, Bainbridge JB et al. Gene therapy for retinitis pigmentosa and Leber congenital amaurosis caused by defects in AIPL1: effective rescue of mouse models of partial and complete Aipl1 deficiency using AAV2/2 and AAV2/8 vectors. Hum Mol Genet 2009; 18: 2099–2114.
Doria M, Ferrara A, Auricchio A . AAV2/8 vectors purified from culture medium with a simple and rapid protocol transduce murine liver, muscle, and retina efficiently. Hum Gene Ther Methods 2013; 24: 392–398.
Drittanti L, Rivet C, Manceau P, Danos O, Vega M . High throughput production, screening and analysis of adeno-associated viral vectors. Gene Therapy 2000; 7: 924–929.
Li A, Zhu X, Craft CM . Retinoic acid upregulates cone arrestin expression in retinoblastoma cells through a Cis element in the distal promoter region. Invest Ophthalmol Vis Sci 2002; 43: 1375–1383.
Acknowledgements
We thank Annamaria Carissimo (Bioinformatics Core, TIGEM, Naples, Italy) for the statistical analyses; Enrico M Surace (TIGEM, Naples, Italy) and Elena Marrocco (TIGEM, Naples, Italy) for support with work in pigs; Monica Doria and Antonella Ferrara (AAV Vector Core, TIGEM, Naples, Italy) for AAV vector production; Mary D Allen (Laboratory for Vision Research, Doheny Eye Institute) and Dr Cheryl MCraft (University of Southern California, Los Angeles, CA) for providing the anti-human CAR antibody; Graciana Diez-Roux (Scientific Office, TIGEM, Naples, Italy) for the critical reading of this manuscript. This work was supported by: the European Research Council/ERC Grant agreement no. 282085 ‘RetGeneTx’; the European Community's Seventh Framework Program [FP7/2007–2013] under Grant agreement no. 242013 ‘Treatrush’; the NIH (grant R24 RY019861-01A); the Italian Telethon Foundation (grant TGM11MT1).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Supplementary Information accompanies this paper on Gene Therapy website
Supplementary information
Rights and permissions
About this article
Cite this article
Colella, P., Trapani, I., Cesi, G. et al. Efficient gene delivery to the cone-enriched pig retina by dual AAV vectors. Gene Ther 21, 450–456 (2014). https://doi.org/10.1038/gt.2014.8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/gt.2014.8
This article is cited by
-
Development of a dual hybrid AAV vector for endothelial-targeted expression of von Willebrand factor
Gene Therapy (2023)
-
mRNA trans-splicing dual AAV vectors for (epi)genome editing and gene therapy
Nature Communications (2023)
-
CRISPR/Cas9 editing of the MYO7A gene in rhesus macaque embryos to generate a primate model of Usher syndrome type 1B
Scientific Reports (2022)
-
Therapeutic homology-independent targeted integration in retina and liver
Nature Communications (2022)
-
New frontiers to cure Alport syndrome: COL4A3 and COL4A5 gene editing in podocyte-lineage cells
European Journal of Human Genetics (2020)