Abstract
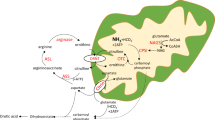
Hyperammonemia is less severe in arginase 1 deficiency compared with other urea cycle defects. Affected patients manifest hyperargininemia and infrequent episodes of hyperammonemia. Patients typically suffer from neurological impairment with cortical and pyramidal tract deterioration, spasticity, loss of ambulation, seizures and intellectual disability; death is less common than with other urea cycle disorders. In a mouse model of arginase I deficiency, the onset of symptoms begins with weight loss and gait instability, which progresses toward development of tail tremor with seizure-like activity; death typically occurs at about 2 weeks of life. Adeno-associated viral vector gene replacement strategies result in long-term survival of mice with this disorder. With neonatal administration of vector, the viral copy number in the liver greatly declines with hepatocyte proliferation in the first 5 weeks of life. Although the animals do survive, it is not known from a functional standpoint how well the urea cycle is functioning in the adult animals that receive adeno-associated virus. In these studies, we administered [1-13C] acetate to both littermate controls and adeno-associated virus-treated arginase 1 knockout animals and examined flux through the urea cycle. Circulating ammonia levels were mildly elevated in treated animals. Arginine and glutamine also had perturbations. Assessment 30 min after acetate administration demonstrated that ureagenesis was present in the treated knockout liver at levels as low at 3.3% of control animals. These studies demonstrate that only minimal levels of hepatic arginase activity are necessary for survival and ureagenesis in arginase-deficient mice and that this level of activity results in control of circulating ammonia. These results may have implications for potential therapy in humans with arginase deficiency.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Summar M, Tuchman M . Proceedings of a consensus conference for the management of patients with urea cycle disorders. J Pediatr 2001; 138: S6–10.
Batshaw ML, Brusilow S, Waber L, Blom W, Brubakk AM, Burton BK et al. Treatment of inborn errors of urea synthesis: activation of alternative pathways of waste nitrogen synthesis and excretion. N Engl J Med 1982; 306: 1387–1392.
Prasad AN, Breen JC, Ampola MG, Rosman NP . Argininemia: a treatable genetic cause of progressive spastic diplegia simulating cerebral palsy: case reports and literature review. J Child Neurol 1997; 12: 301–309.
Jain-Ghai S, Nagamani SC, Blaser S, Siriwardena K, Feigenbaum A . Arginase I deficiency: severe infantile presentation with hyperammonemia: more common than reported? Mol Genet Metab 2011; 104: 107–111.
Picker JD, Puga AC, Levy HL, Marsden D, Shih VE, Degirolami U et al. Arginase deficiency with lethal neonatal expression: evidence for the glutamine hypothesis of cerebral edema. J Pediatr 2003; 142: 349–352.
Lee EK, Hu C, Bhargava R, Rozengurt N, Stout D, Grody WW et al. Long-term survival of the juvenile lethal arginase-deficient mouse with AAV gene therapy. Mol Therapy 2012; 20: 1844–1851.
Lee EK, Hu C, Bhargava R, Ponnusamy R, Park H, Novicoff S et al. AAV-based gene therapy prevents neuropathology and results in normal cognitive development in the hyperargininemic mouse. Gene Therapy 2013; 20: 785–796.
Hu C, Kasten J, Park H, Bhargava R, Tai DS, Grody WW et al. Myocyte-mediated arginase expression controls hyperargininemia but not hyperammonemia in arginase-deficient mice. Mol Therapy 2014; 22: 1792–1802.
Hu C, Busuttil RW, Lipshutz GS . RH10 provides superior transgene expression in mice when compared with natural AAV serotypes for neonatal gene therapy. J Gene Med 2010; 12: 766–778.
Hu C, Lipshutz GS . AAV-based neonatal gene therapy for hemophilia A: long-term correction and avoidance of immune responses in mice. Gene Ther 2012; 19: 1166–1176.
Yudkoff M, Daikhin Y, Ye X, Wilson JM, Batshaw ML . In vivo measurement of ureagenesis with stable isotopes. J Inherit Metab Dis 1998; 21: 21–29.
Iyer RK, Yoo PK, Kern RM, Rozengurt N, Tsoa R, O'Brien WE et al. Mouse model for human arginase deficiency. Mol Cell Biol 2002; 22: 4491–4498.
Mew NA, Yudkoff M, Tuchman M . Stable isotopes in the diagnosis and treatment of inherited hyperammonemia. J Pediatr Biochem 2014; 4: 57–63.
Yudkoff M, Ah Mew N, Daikhin Y, Horyn O, Nissim I, Payan I et al. Measuring in vivo ureagenesis with stable isotopes. Mol Genet Metab 2010; 100: S37–S41.
Ah Mew N, Payan I, Daikhin Y, Nissim I, Tuchman M, Yudkoff M . Effects of a single dose of N-carbamylglutamate on the rate of ureagenesis. Mol Genet Metab 2009; 98: 325–330.
Tuchman M, Caldovic L, Daikhin Y, Horyn O, Nissim I, Korson M et al. N-carbamylglutamate markedly enhances ureagenesis in N-acetylglutamate deficiency and propionic acidemia as measured by isotopic incorporation and blood biomarkers. Pediatr Res 2008; 64: 213–217.
Caldovic L, Morizono H, Daikhin Y, Nissim I, McCarter RJ, Yudkoff M et al. Restoration of ureagenesis in N-acetylglutamate synthase deficiency by N-carbamylglutamate. J Pediatr 2004; 145: 552–554.
Gau CL, Rosenblatt RA, Cerullo V, Lay FD, Dow AC, Livesay J et al. Short-term correction of arginase deficiency in a neonatal murine model with a helper-dependent adenoviral vector. Mol Ther 2009; 17: 1155–1163.
Miller RE, Pope SR, DeWille JW, Burns DM . Insulin decreases and hydrocortisone increases the synthesis of glutamine synthetase in cultured 3T3-L1 adipocytes. J Biol Chem 1983; 258: 5405–5413.
Moscioni D, Morizono H, McCarter RJ, Stern A, Cabrera-Luque J, Hoang A et al. Long-term correction of ammonia metabolism and prolonged survival in ornithine transcarbamylase-deficient mice following liver-directed treatment with adeno-associated viral vectors. Mol Ther 2006; 14: 25–33.
Cunningham SC, Spinoulas A, Carpenter KH, Wilcken B, Kuchel PW, Alexander IE . AAV2/8-mediated correction of OTC deficiency is robust in adult but not neonatal Spf(ash) mice. Mol Ther 2009; 17: 1340–1346.
Kok CY, Cunningham SC, Carpenter KH, Dane AP, Siew SM, Logan GJ et al. Adeno-associated virus-mediated rescue of neonatal lethality in argininosuccinate synthetase-deficient mice. Mol Ther 2013; 21: 1823–1831.
Kasten J, Hu C, Bhargava R, Park H, Tai D, Byrne JA et al. Lethal phenotype in conditional late-onset arginase 1 deficiency in the mouse. Mol Genet Metab 2013; 110: 222–230.
Jones BN, Gilligan JP . o-Phthaldialdehyde precolumn derivatization and reversed-phase high-performance liquid chromatography of polypeptide hydrolysates and physiological fluids. J Chromatogr 1983; 266: 471–482.
Acknowledgements
We thank Ilana Nissim and Evgueni Daikhin for performing the ureagenesis analysis and both the Semel Institute for Neuroscience and the Intellectual and Developmental Disabilities Research Center at UCLA for their support. This work was supported by grants from the National Institutes of Health to GSL (1R01NS071076-04A1 and 1R01NS071076-04S) and was supported by the Metabolomic Core at the Children's Hospital of Philadelphia and by grant HD26979 from the Eunice Kennedy Shriver Institute of Child Health of the NIH. DST received funding from the Society of University Surgeons and was a recipient of a NIGMS Medical Genetics NIHT32 (GM008243).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Rights and permissions
About this article
Cite this article
Hu, C., Tai, D., Park, H. et al. Minimal ureagenesis is necessary for survival in the murine model of hyperargininemia treated by AAV-based gene therapy. Gene Ther 22, 111–115 (2015). https://doi.org/10.1038/gt.2014.106
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/gt.2014.106
This article is cited by
-
Argininosuccinic aciduria fosters neuronal nitrosative stress reversed by Asl gene transfer
Nature Communications (2018)
-
Delivering efficient liver-directed AAV-mediated gene therapy
Gene Therapy (2017)
-
Restoring Ureagenesis in Hepatocytes by CRISPR/Cas9-mediated Genomic Addition to Arginase-deficient Induced Pluripotent Stem Cells
Molecular Therapy - Nucleic Acids (2016)