Abstract
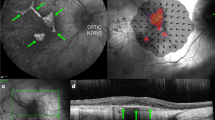
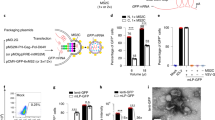
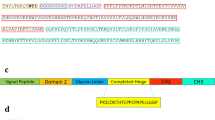
Inhibition of vascular endothelial growth factor (VEGF) has become the standard of care for patients presenting with wet age-related macular degeneration. However, monthly intravitreal injections are required for optimal efficacy. We have previously shown that electroporation enabled ciliary muscle gene transfer results in sustained protein secretion into the vitreous for up to 9 months. Here, we evaluated the long-term efficacy of ciliary muscle gene transfer of three soluble VEGF receptor-1 (sFlt-1) variants in a rat model of laser-induced choroidal neovascularization (CNV). All three sFlt-1 variants significantly diminished vascular leakage and neovascularization as measured by fluorescein angiography (FA) and flatmount choroid at 3 weeks. FA and infracyanine angiography demonstrated that inhibition of CNV was maintained for up to 6 months after gene transfer of the two shortest sFlt-1 variants. Throughout, clinical efficacy was correlated with sustained VEGF neutralization in the ocular media. Interestingly, treatment with sFlt-1 induced a 50% downregulation of VEGF messenger RNA levels in the retinal pigment epithelium and the choroid. We demonstrate for the first time that non-viral gene transfer can achieve a long-term reduction of VEGF levels and efficacy in the treatment of CNV.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
References
Dhoot DS, Kaiser PK . Ranibizumab for age-related macular degeneration. Expert Opin Biol Ther 2012; 12: 371–381.
Browning DJ, Kaiser PK, Rosenfeld PJ, Stewart MW . Aflibercept for age-related macular degeneration: a game-changer or quiet addition? Am J Ophthalmol 2012; 154: 222–226.
Ohr M, Kaiser PK . Intravitreal aflibercept injection for neovascular (wet) age-related macular degeneration. Expert Opin Pharmacother 2012; 13: 585–591.
Muether PS, Hermann MM, Viebahn U, Kirchhof B, Fauser S . Vascular endothelial growth factor in patients with exudative age-related macular degeneration treated with ranibizumab. Ophthalmology 2012; 119: 2082–2086.
Campochiaro PA . Anti-vascular endothelial growth factor treatment for retinal vein occlusions. Ophthalmologica 2012; 227 (Suppl 1): 30–35.
Ho AC, Scott IU, Kim SJ, Brown GC, Brown MM, Ip MS et al. Anti-vascular endothelial growth factor pharmacotherapy for diabetic macular edema: a report by the american academy of ophthalmology. Ophthalmology 2012; 119: 2179–2188.
Zechmeister-Koss I, Huic M . Vascular endothelial growth factor inhibitors (anti-VEGF) in the management of diabetic macular oedema: a systematic review. Br J Ophthalmol 2012; 96: 167–178.
El Sanharawi M, Kowalczuk L, Touchard E, Omri S, de Kozak Y, Behar-Cohen F . Protein delivery for retinal diseases: from basic considerations to clinical applications. Prog Retin Eye Res 2010; 29: 443–465.
Cideciyan AV . Leber congenital amaurosis due to RPE65 mutations and its treatment with gene therapy. Prog Retin Eye Res 2010; 29: 398–427.
Hufnagel RB, Ahmed ZM, Correa ZM, Sisk RA . Gene therapy for Leber congenital amaurosis: advances and future directions. Graefes Arch Clin Exp Ophthalmol 2012; 250: 1117–1128.
Smith AJ, Bainbridge JW, Ali RR . Gene supplementation therapy for recessive forms of inherited retinal dystrophies. Gene Ther 2012; 19: 154–161.
Bainbridge JW, Mistry A, De Alwis M, Paleolog E, Baker A, Thrasher AJ et al. Inhibition of retinal neovascularisation by gene transfer of soluble VEGF receptor sFlt-1. Gene Ther 2002; 9: 320–326.
Ideno J, Mizukami H, Kakehashi A, Saito Y, Okada T, Urabe M et al. Prevention of diabetic retinopathy by intraocular soluble flt-1 gene transfer in a spontaneously diabetic rat model. Int J Mol Med 2007; 19: 75–79.
Mao Y, Kiss S, Boyer JL, Hackett NR, Qiu J, Carbone A et al. Persistent suppression of ocular neovascularization with intravitreal administration of AAVrh.10 coding for bevacizumab. Hum Gene Ther 2011; 22: 1525–1535.
Pechan P, Rubin H, Lukason M, Ardinger J, DuFresne E, Hauswirth WW et al. Novel anti-VEGF chimeric molecules delivered by AAV vectors for inhibition of retinal neovascularization. Gene Ther 2009; 16: 10–16.
Provost N, Le Meur G, Weber M, Mendes-Madeira A, Podevin G, Cherel Y et al. Biodistribution of rAAV vectors following intraocular administration: evidence for the presence and persistence of vector DNA in the optic nerve and in the brain. Mol Ther 2005; 11: 275–283.
Stieger K, Schroeder J, Provost N, Mendes-Madeira A, Belbellaa B, Le Meur G et al. Detection of intact rAAV particles up to 6 years after successful gene transfer in the retina of dogs and primates. Mol Ther 2009; 17: 516–523.
Tao W . Application of encapsulated cell technology for retinal degenerative diseases. Expert Opin Biol Ther 2006; 6: 717–726.
Touchard E, Kowalczuk L, Bloquel C, Naud MC, Bigey P, Behar-Cohen F . The ciliary smooth muscle electrotransfer: basic principles and potential for sustained intraocular production of therapeutic proteins. J Gene Med 2010; 12: 904–919.
de Vries C, Escobedo JA, Ueno H, Houck K, Ferrara N, Williams LT . The fms-like tyrosine kinase, a receptor for vascular endothelial growth factor. Science 1992; 255: 989–991.
Mustonen T, Alitalo K . Endothelial receptor tyrosine kinases involved in angiogenesis. J Cell Biol 1995; 129: 895–898.
He Y, Smith SK, Day KA, Clark DE, Licence DR, Charnock-Jones DS . Alternative splicing of vascular endothelial growth factor (VEGF)-R1 (FLT-1) pre-mRNA is important for the regulation of VEGF activity. Mol Endocrinol 1999; 13: 537–545.
Kendall RL, Thomas KA . Inhibition of vascular endothelial cell growth factor activity by an endogenously encoded soluble receptor. Proc Natl Acad Sci USA 1993; 90: 10705–10709.
Kendall RL, Wang G, Thomas KA . Identification of a natural soluble form of the vascular endothelial growth factor receptor, FLT-1, and its heterodimerization with KDR. Biochem Biophys Res Commun 1996; 226: 324–328.
Barleon B, Totzke F, Herzog C, Blanke S, Kremmer E, Siemeister G et al. Mapping of the sites for ligand binding and receptor dimerization at the extracellular domain of the vascular endothelial growth factor receptor FLT-1. J Biol Chem 1997; 272: 10382–10388.
Cunningham SA, Stephan CC, Arrate MP, Ayer KG, Brock TA . Identification of the extracellular domains of Flt-1 that mediate ligand interactions. Biochem Biophys Res Commun 1997; 231: 596–599.
Basile DP, Fredrich K, Chelladurai B, Leonard EC, Parrish AR . Renal ischemia reperfusion inhibits VEGF expression and induces ADAMTS-1, a novel VEGF inhibitor. Am J Physiol Renal Physiol 2008; 294: F928–F936.
Gehlbach P, Demetriades AM, Yamamoto S, Deering T, Xiao WH, Duh EJ et al. Periocular gene transfer of sFlt-1 suppresses ocular neovascularization and vascular endothelial growth factor-induced breakdown of the blood-retinal barrier. Hum Gene Ther 2003; 14: 129–141.
Igarashi T, Miyake K, Masuda I, Takahashi H, Shimada T . Adeno-associated vector (type 8)-mediated expression of soluble Flt-1 efficiently inhibits neovascularization in a murine choroidal neovascularization model. Hum Gene Ther 2010; 21: 631–637.
Lai CM, Shen WY, Brankov M, Lai YK, Barnett NL, Lee SY et al. Long-term evaluation of AAV-mediated sFlt-1 gene therapy for ocular neovascularization in mice and monkeys. Mol Ther 2005; 12: 659–668.
Lai YK, Shen WY, Brankov M, Lai CM, Constable IJ, Rakoczy PE . Potential long-term inhibition of ocular neovascularisation by recombinant adeno-associated virus-mediated secretion gene therapy. Gene Ther 2002; 9: 804–813.
Edelman JL, Castro MR . Quantitative image analysis of laser-induced choroidal neovascularization in rat. Exp Eye Res 2007; 71: 523–533.
Kaiser PK . Strategies for inhibiting vascular endothelial growth factor. Retina 2009; 29: S15–S17.
Antonetti DA, Klein R, Gardner TW . Diabetic retinopathy. N Engl J Med 2012; 366: 1227–1239.
Ohno-Matsui K, Hirose A, Yamamoto S, Saikia J, Okamoto N, Gehlbach P et al. Inducible expression of vascular endothelial growth factor in adult mice causes severe proliferative retinopathy and retinal detachment. Am J Pathol 2002; 160: 711–719.
Okamoto N, Tobe T, Hackett SF, Ozaki H, Vinores MA, LaRochelle W et al. Transgenic mce with increased expression of vascular endothelial growth factor in the retina: a new model of intraretinal and subretinal neovascularization. Am J Pathol 1997; 151: 281–291.
Yamada E, Tobe T, Yamada H, Okamoto N, Zack DJ, Werb Z et al. TIMP-1 promotes VEGF-induced neovascularization in the retina. Histol Histopathol 2001; 16: 87–97.
Kovach JL, Schwartz SG, Flynn HW Jr., Scott IU . Anti-VEGF treatment strategies for wet AMD. J Ophthalmol 2012; 2012: 786870.
Kowalczuk L, Touchard E, Omri S, Jonet L, Klein C, Valamanes F et al. Placental growth factor contributes to micro-vascular abnormalization and blood-retinal barrier breakdown in diabetic retinopathy. PLoS One 2011; 6: e17462.
Miyamoto N, de Kozak Y, Jeanny JC, Glotin A, Mascarelli F, Massin P et al. Placental growth factor-1 and epithelial haemato-retinal barrier breakdown: potential implication in the pathogenesis of diabetic retinopathy. Diabetologia 2007; 50: 461–470.
Miyamoto N, de Kozak Y, Normand N, Courtois Y, Jeanny JC, Benezra D et al. PlGF-1 and VEGFR-1 pathway regulation of the external epithelial hemato-ocular barrier. A model for retinal edema. Ophthalmic Res 2008; 40: 203–207.
Marano RJ, Toth I, Wimmer N, Brankov M, Rakoczy PE . Dendrimer delivery of an anti-VEGF oligonucleotide into the eye: a long-term study into inhibition of laser-induced CNV, distribution, uptake and toxicity. Gene Ther 2005; 12: 1544–1550.
Lukason M, DuFresne E, Rubin H, Pechan P, Li Q, Kim I et al. Inhibition of choroidal neovascularization in a nonhuman primate model by intravitreal administration of an AAV2 vector expressing a novel anti-VEGF molecule. Mol Ther 2011; 19: 260–265.
Rosenfeld PJ, Brown DM, Heier JS, Boyer DS, Kaiser PK, Chung CY et alMARINA Study Group. Ranibizumab for neovascular age-related macular degeneration. N Engl J Med 2006; 355: 1419–1431.
Saint-Geniez M, Kurihara T, Sekiyama E, Maldonado AE, D’Amore PA . An essential role for RPE-derived soluble VEGF in the maintenance of the choriocapillaris. Proc Natl Acad Sci USA 2009; 106: 18751–18756.
Lois N, McBain V, Abdelkader E, Scott NW, Kumari R . Retinal pigment epithelial atrophy in patients with exudative age-related macular degeneration undergoing anti-vascular endothelial growth factor therapy. Retina 2013; 33: 13–22.
Touchard E, Heiduschka P, Berdugo M, Kowalczuk L, Bigey P, Chahory S et al. Non-viral gene therapy for GDNF production in RCS rat: the crucial role of the plasmid dose. Gene Ther 2012; 19: 886–898.
Touchard E, Bloquel C, Bigey P, Kowalczuk L, Jonet L, Thillaye-Goldenberg B et al. Effects of ciliary muscle plasmid electrotransfer of TNF-alpha soluble receptor variants in experimental uveitis. Gene Ther 2009; 16: 862–873.
Drechsler F, Koferl P, Hollborn M, Wiedemann P, Bringmann A, Kohen L et al. Effect of intravitreal anti-vascular endothelial growth factor treatment on the retinal gene expression in acute experimental central retinal vein occlusion. Ophthalmic Res 2012; 47: 157–162.
Xu B, Thornton C, Tooher J, Hennessy A . Exogenous soluble VEGF receptor-1 (sFlt-1) regulates Th1/Th2 cytokine production from normal placental explants via intracellular calcium. Hypertens Pregnancy 2009; 28: 448–456.
Rocha FG, Calvo FB, Chaves KC, Peron JP, Marques RF, de Borba TR et al. Endostatin- and interleukin-2-expressing retroviral bicistronic vector for gene therapy of metastatic renal cell carcinoma. J Gene Med 2011; 13: 148–157.
Alì G, Boldrini L, Lucchi M, Picchi A, Dell’Omodarme M, Prati MC et al. Treatment with interleukin-2 in malignant pleural mesothelioma: immunological and angiogenetic assessment and prognostic impact. Br J Cancer 2009; 101: 1869–1875.
Girard S, Larouche A, Kadhim H, Rola-Pleszczynski M, Gobeil F, Sébire G . Lipopolysaccharide and hypoxia/ischemia induced IL-2 expression by microglia in neonatal brain. Neuroreport 2008; 19: 997–1002.
Roh MI, Kim HS, Song JH, Lim JB, Koh HJ, Kwon OW . Concentration of cytokines in the aqueous humor of patients with naive, recurrent and regressed CNV associated with amd after bevacizumab treatment. Retina 2009; 29: 523–529.
Ryu JK, Cho T, Choi HB, Wang YT, McLarnon JG . Microglial VEGF receptor response is an integral chemotactic component in Alzheimer’s disease pathology. J Neurosci 2009; 29: 3–13.
Vinores SA, Xiao WH, Zimmerman R, Whitcup SM, Wawrousek EF . Upregulation of vascular endothelial growth factor (VEGF) in the retinas of transgenic mice overexpressing interleukin-1beta (IL-1beta) in the lens and mice undergoing retinal degeneration. Histol Histopathol 2003; 18: 797–810.
Khoury M, Bigey P, Louis-Plence P, Noel D, Rhinn H, Scherman D et al. A comparative study on intra-articular versus systemic gene electrotransfer in experimental arthritis. J Gene Med 2006; 8: 1027–1036.
Wiesmann C, Fuh G, Christinger HW, Eigenbrot C, Wells JA, de Vos AM . Crystal structure at 1.7A resolution of VEGF in complex with domain 2 of the Flt-1 receptor. Cell 1997; 91: 695–704.
Pfaffl MW . A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res 2001; 29: e45.
Shibuya M, Yamaguchi S, Yamane A, Ikeda T, Tojo A, Matsushime H et al. Nucleotide sequence and expression of a novel human receptor-type tyrosine kinase gene (flt) closely related to the fms family. Oncogene 1990; 5: 519–524.
Acknowledgements
This work was funded in part by Fondation pour la Recherche Médicale (FRM).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Rights and permissions
About this article
Cite this article
El Sanharawi, M., Touchard, E., Benard, R. et al. Long-term efficacy of ciliary muscle gene transfer of three sFlt-1 variants in a rat model of laser-induced choroidal neovascularization. Gene Ther 20, 1093–1103 (2013). https://doi.org/10.1038/gt.2013.36
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/gt.2013.36