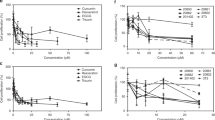
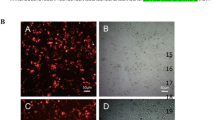
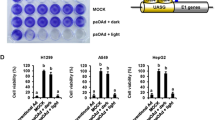
Abstract
High-risk Human Papillomaviruses (HPV) has been found to be associated with carcinomas of the cervix, penis, vulva/vagina, anus, mouth and oro-pharynx. As the main tumorigenic effects of the HPV have been attributed to the expression of E6 and E7 genes, different gene therapy approaches have been directed to block their expression such as antisense oligonucleotides (ASO), ribozymes and small interfering RNAs. In order to develop a gene-specific therapy for HPV-related cancers, we investigated a potential therapeutic strategy of gene silencing activated under illumination. Our aim according to this antisense therapy consisted in regulating the HPV16 E6 oncogene by using an E6-ASO derivatized with a polyazaaromatic ruthenium (RuII) complex (E6-Ru-ASO) able, under visible illumination, to crosslink irreversibly the targeted sequence. We examined the effects of E6-Ru-ASO on the expression of E6 and on the cell growth of cervical cancer cells. We demonstrated using HPV16+ SiHa cervical cancer cells that E6-Ru-ASO induces after illumination, a reactivation of p53, the most important target of E6, as well as the inhibition of cell proliferation with a selective repression of E6 at the protein level. These results suggest that E6-Ru ASOs, activated under illumination and specifically targeting E6, are capable of inhibiting HPV16+ cervical cancer cell proliferation.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
References
Munger K, Baldwin A, Edwards KM, Hayakawa H, Nguyen CL, Owens M et al. Mechanisms of human papillomavirus-induced oncogenesis. J Virol 2004; 78: 11451–11460.
zur Hausen H . Papillomaviruses and cancer: from basic studies to clinical application. Nat Rev Cancer 2002; 2: 342–350.
Campion MJ . Clinical manifestations and natural history of genital human papillomavirus infection. Obstet Gynecol Clin North Am 1987; 14: 363–388.
Syrjanen KJ . Biology of human papillomavirus (HPV) infections and their role in squamous cell carcinogenesis. Med Biol 1987; 65: 21–39.
Parkin DM . The global health burden of infection-associated cancers in the year 2002. Int J Cancer 2006; 118: 3030–3044.
Cullen AP, Reid R, Campion M, Lorincz AT . Analysis of the physical state of different human papillomavirus DNAs in intraepithelial and invasive cervical neoplasm. J Virol 1991; 65: 606–612.
Daling JR, Madeleine MM, Johnson LG, Schwartz SM, Shera KA, Wurscher MA et al. Human papillomavirus, smoking, and sexual practices in the etiology of anal cancer. Cancer 2004; 101: 270–280.
Lorincz AT, Temple GF, Kurman RJ, Jenson AB, Lancaster WD . Oncogenic association of specific human papillomavirus types with cervical neoplasia. J Natl Cancer Inst 1987; 79: 671–677.
Miller CS, Johnstone BM . Human papillomavirus as a risk factor for oral squamous cell carcinoma: a meta-analysis, 1982-1997. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 2001; 91: 622–635.
Walboomers JM, Jacobs MV, Manos MM, Bosch FX, Kummer JA, Shah KV et al. Human papillomavirus is a necessary cause of invasive cervical cancer worldwide. J Pathol 1999; 189: 12–19.
Shillitoe EJ . Papillomaviruses as targets for cancer gene therapy. Cancer Gene Ther 2006; 13: 445–450.
Schmook T, Stockfleth E . Current treatment patterns in non-melanoma skin cancer across Europe. J Dermatolog Treat 2003; 14 (Suppl 3): 3–10.
Rossi R, Bruscino N, Ricceri F, Grazzini M, Dindelli M, Lotti T . Photodynamic treatment for viral infections of the skin. Giornale Italiano di Dermatologia e Venereologia 2009; 144: 79–83.
Kubler AC, Haase T, Staff C, Kahle B, Rheinwald M, Muhling J . Photodynamic therapy of primary nonmelanomatous skin tumours of the head and neck. Lasers Surg Med 1999; 25: 60–68.
Petrelli NJ, Cebollero JA, Rodriguez-Bigas M, Mang T . Photodynamic therapy in the management of neoplasms of the perianal skin. Arch Surg 1992; 127: 1436–1438.
Butz K, Ristriani T, Hengstermann A, Denk C, Scheffner M, Hoppe-Seyler F . siRNA targeting of the viral E6 oncogene efficiently kills human papillomavirus-positive cancer cells. Oncogene 2003; 22: 5938–5945.
Chi KN, Eisenhauer E, Fazli L, Jones EC, Goldenberg SL, Powers J et al. A phase I pharmacokinetic and pharmacodynamic study of OGX-011, a 2'-methoxyethyl antisense oligonucleotide to clusterin, in patients with localized prostate cancer. J Natl Cancer Inst 2005; 97: 1287–1296.
Crooke ST . Antisense strategies. Curr Mol Med 2004; 4: 465–487.
Hong D, Lu W, Ye F, Hu Y, Xie X . Gene silencing of HPV16 E6/E7 induced by promoter-targeting siRNA in SiHa cells. Br J Cancer 2009; 101: 1798–1804.
Jiang M, Milner J . Selective silencing of viral gene expression in HPV-positive human cervical carcinoma cells treated with siRNA, a primer of RNA interference. Oncogene 2002; 21: 6041–6048.
Koivusalo R, Mialon A, Pitkanen H, Westermarck J, Hietanen S . Activation of p53 in cervical cancer cells by human papillomavirus E6 RNA interference is transient, but can be sustained by inhibiting endogenous nuclear export-dependent p53 antagonists. Cancer Res 2006; 66: 11817–11824.
Yamayoshi A, Kato K, Suga S, Ichinoe A, Arima T, Matsuda T et al. Specific apoptosis induction in human papillomavirus-positive cervical carcinoma cells by photodynamic antisense regulation. Oligonucleotides 2007; 17: 66–79.
Zhang J, Hua ZC . Targeted gene silencing by small interfering RNA-based knock-down technology. Curr Pharm Biotechnol 2004; 5: 1–7.
Elias B, Kirsch-De Mesmaeker A . Photo-reduction of polyazaaromatic Ru(II) complexes by biomolecules and possible applications. Coordination Chem Rev 2006; 250: 1627–1641.
Herman L, Ghosh S, Defrancq E, Kirsch-De Mesmaeker A . Ru(II) complexes and light: molecular tools for biomolecules. J Phys Org Chem 2008; 21: 670–681.
Lentzen O, Defrancq E, Constant JF, Schumm S, Garcia-Fresnadillo D, Moucheron C et al. Determination of DNA guanine sites forming photo-adducts with Ru(II)-labeled oligonucleotides; DNA polymerase inhibition by the resulting photo-crosslinking. J Biol Inorg Chem 2004; 9: 100–108.
Lentzen O, Constant JF, Defrancq E, Prevost M, Schumm S, Moucheron C et al. Photocrosslinking in ruthenium-labelled duplex oligonucleotides. Chem Bio Chem 2003; 4: 195–202.
Le Gac S, Rickling S, Gerbaux P, Defrancq E, Moucheron C, Kirsch-De Mesmaeker A . A photoreactive ruthenium(II) complex tethered to a guanine-containing oligonucleotide: a biomolecular tool that behaves as a "seppuku molecule". Angew Chem Int Ed Engl 2009; 48: 1122–1125.
Le Gac S, Foucart M, Gerbaux P, Defrancq E, Moucheron C, Kirsch-De Mesmaeker A . Photo-reactive Ru(II)-oligonucleotide conjugates: influence of an intercalating ligand on the inter- and intra-strand photo-ligation processes. Dalton Transactions 2010; 39: 9672–9683.
Brown SB, Brown EA, Walker I . The present and future role of photodynamic therapy in cancer treatment. Lancet Oncol 2004; 5: 497–508.
DeFilippis RA, Goodwin EC, Wu L, Dimaio D . Endogenous human papillomavirus E6 and E7 proteins differentially regulate proliferation, senescence, and apoptosis in HeLa cervical carcinoma cells. J Virol 2003; 77: 1551–1563.
Goodwin EC, Dimaio D . Repression of human papillomavirus oncogenes in HeLa cervical carcinoma cells causes the orderly reactivation of dormant tumor suppressor pathways. Proc Natl Acad Sci USA 2000; 97: 12513–1258.
Lechner MS, Mack DH, Finicle AB, Crook T, Vousden KH, Laimins LA . Human papillomavirus E6 proteins bind p53 in vivo and abrogate p53-mediated repression of transcription. EMBO J 1992; 11: 3045–3052.
Thomas M, Pim D, Banks L . The role of the E6-p53 interaction in the molecular pathogenesis of HPV. Oncogene 1999; 18: 7690–7700.
Hietanen S, Lain S, Krausz E, Blattner C, Lane DP . Activation of p53 in cervical carcinoma cells by small molecules. Proc Natl Acad Sci USA 2000; 97: 8501–8506.
Koivusalo R, Hietanen S . The cytotoxicity of chemotherapy drugs varies in cervical cancer cells depending on the p53 status. Cancer Biol Ther 2004; 3: 1177–1183.
Schiller JT, Lowy DR . Prospects for cervical cancer prevention by human papillomavirus vaccination. Cancer Res 2006; 66: 10229–1032.
Wright TC, Van DP, Schmitt HJ, Meheus A . Chapter 14: HPV vaccine introduction in industrialized countries. Vaccine 2006; 24 (Suppl 3): S3-122–S3-131.
Gilbert DJ . Treatment of actinic keratoses with sequential combination of 5-fluorouracil and photodynamic therapy. J Drugs Dermatol 2005; 4: 161–163.
Morton C, Campbell S, Gupta G, Keohane S, Lear J, Zaki I et al. Intraindividual, right-left comparison of topical methyl aminolaevulinate-photodynamic therapy and cryotherapy in subjects with actinic keratoses: a multicentre, randomized controlled study. Br J Dermatol 2006; 155: 1029–1036.
Piacquadio DJ, Chen DM, Farber HF, Fowler JF, Glazer SD, Goodman JJ et al. Photodynamic therapy with aminolevulinic acid topical solution and visible blue light in the treatment of multiple actinic keratoses of the face and scalp: investigator-blinded, phase 3, multicenter trials. Arch Dermatol 2004; 140: 41–46.
Tschen EH, Wong DS, Pariser DM, Dunlap FE, Houlihan A, Ferdon MB . Photodynamic therapy using aminolaevulinic acid for patients with nonhyperkeratotic actinic keratoses of the face and scalp: phase IV multicentre clinical trial with 12-month follow up. Br J Dermatol 2006; 155: 1262–1269.
Dijkstra AT, Majoie IM, van Dongen JW, van WH, van Vloten WA . Photodynamic therapy with violet light and topical 6-aminolaevulinic acid in the treatment of actinic keratosis, Bowen's disease and basal cell carcinoma. J Eur Acad 2001; 15: 550–554.
Morton C, Horn M, Leman J, Tack B, Bedane C, Tjioe M et al. Comparison of topical methyl aminolevulinate photodynamic therapy with cryotherapy or Fluorouracil for treatment of squamous cell carcinoma in situ: results of a multicenter randomized trial. Arch Dermatol 2006; 142: 729–735.
Morton CA, Whitehurst C, Moore JV, MacKie RM . Comparison of red and green light in the treatment of Bowen's disease by photodynamic therapy. Br J Dermatol 2000; 143: 767–772.
Basset-Seguin N, Ibbotson SH, Emtestam L, Tarstedt M, Morton C, Maroti M et al. Topical methyl aminolaevulinate photodynamic therapy versus cryotherapy for superficial basal cell carcinoma: a 5 year randomized trial. Eur J Dermatol 2008; 18: 547–553.
Rhodes LE, de Rie MA, Leifsdottir R, Yu RC, Bachmann I, Goulden V et al. Five-year follow-up of a randomized, prospective trial of topical methyl aminolevulinate photodynamic therapy vs surgery for nodular basal cell carcinoma. Arch Dermatol 2007; 143: 1131–1136.
Vinciullo C, Elliott T, Francis D, Gebauer K, Spelman L, Nguyen R et al. Photodynamic therapy with topical methyl aminolaevulinate for 'difficult-to-treat' basal cell carcinoma. Br J Dermatol 2005; 152: 765–772.
Morton CA, Brown SB, Collins S, Ibbotson S, Jenkinson H, Kurwa H et al. Guidelines for topical photodynamic therapy: report of a workshop of the British Photodermatology Group. Br J Dermatol 2002; 146: 552–567.
Procianoy F, Cruz AA, Baccega A, Ferraz V, Chahud F . Aggravation of eyelid and conjunctival malignancies following photodynamic therapy in DeSanctis-Cacchione syndrome. Ophthal Plast Reconstr Surg 2006; 22: 498–499.
Wolf P, Rieger E, Kerl H . Topical photodynamic therapy with endogenous porphyrins after application of 5-aminolevulinic acid. An alternative treatment modality for solar keratoses, superficial squamous cell carcinomas, and basal cell carcinomas? J Am Acad Dermatol 1993; 28: 17–21.
Morton CA, McKenna KE, Rhodes LE . Guidelines for topical photodynamic therapy: update. Br J Dermatol 2008; 159: 1245–1266.
Fehr MK, Hornung R, Schwarz VA, Simeon R, Haller U, Wyss P . Photodynamic therapy of vulvar intraepithelial neoplasia III using topically applied 5-aminolevulinic acid. Gynecol Oncol 2001; 80: 62–66.
Fehr MK, Hornung R, Degen A, Schwarz VA, Fink D, Haller U et al. Photodynamic therapy of vulvar and vaginal condyloma and intraepithelial neoplasia using topically applied 5-aminolevulinic acid. Lasers Surg Med 2002; 30: 273–279.
Abdel-Hady ES, Martin-Hirsch P, Duggan-Keen M, Stern PL, Moore JV, Corbitt G et al. Immunological and viral factors associated with the response of vulval intraepithelial neoplasia to photodynamic therapy. Cancer Res 2001; 61: 192–196.
Ascencio M, Collinet P, Cosson M, Vinatier D, Mordon S . [The place of photodynamic therapy in gynecology]. Gynecol Obstet Fertil 2007; 35: 1155–1165.
Antonarakis ES, Emadi A . Ruthenium-based chemotherapeutics: are they ready for prime time? Cancer Chemother Pharmacol 2010; 66: 1–9.
Schatzschneider U, Niesel J, Ott I, Gust R, Alborzinia H, Wolfl S . Cellular uptake, cytotoxicity, and metabolic profiling of human cancer cells treated with ruthenium(II) polypyridyl complexes [Ru(bpy)2(N--N)]Cl2 with N--N=bpy, phen, dpq, dppz, and dppn. Chem Med Chem 2008; 3: 1104–1109.
Kleinpenning MM, Smits T, Frunt MH, van Erp PE, van De Kerkhof PC, Gerritsen RM . Clinical and histological effects of blue light on normal skin. Photodermatol Photoimmunol Photomed 2010; 26: 16–21.
Gicquel E, Boisdenghien A, Defrancq E, Moucheron C, Kirsch-De Mesmaeker A . Adduct formation by photo-induced electron transfer between photo-oxidising Ru(II) complexes and tryptophan. Chem Commun 2004: 2764–2765.
Alvarez-Salas LM, Benitez-Hess ML, DiPaolo JA . Advances in the development of ribozymes and antisense oligodeoxynucleotides as antiviral agents for human papillomaviruses. Antivir Ther 2003; 8: 265–278.
Cho CW, Poo H, Cho YS, Cho MC, Lee KA, Lee SJ et al. HPV E6 antisense induces apoptosis in CaSki cells via suppression of E6 splicing. Exp Mol Med 2002; 34: 159–166.
Venturini F, Braspenning J, Homann M, Gissmann L, Sczakiel G . Kinetic selection of HPV 16 E6/E7-directed antisense nucleic acids: anti-proliferative effects on HPV 16-transformed cells. Nucleic Acids Res 1999; 27: 1585–1592.
Marquez-Gutierrez MA, itez-Hess ML, DiPaolo JA, varez-Salas LM . Effect of combined antisense oligodeoxynucleotides directed against the human papillomavirus type 16 on cervical carcinoma cells. Arch Med Res 2007; 38: 730–738.
Scheffner M, Werness BA, Huibregtse JM, Levine AJ, Howley PM . The E6 oncoprotein encoded by human papillomavirus types 16 and 18 promotes the degradation of p53. Cell 1990; 63: 1129–1136.
Putral LN, Bywater MJ, Gu W, Saunders NA, Gabrielli BG, Leggatt GR et al. RNA interference against human papillomavirus oncogenes in cervical cancer cells results in increased sensitivity to cisplatin. Mol Pharmacol 2005; 68: 1311–1319.
Delvenne P, al-Saleh W, Gilles C, Thiry A, Boniver J . Inhibition of growth of normal and human papillomavirus-transformed keratinocytes in monolayer and organotypic cultures by interferon-gamma and tumor necrosis factor-alpha. Am J Pathol 1995; 146: 589–598.
Hubert P, van den BF, Giannini SL, Franzen-Detrooz E, Boniver J, Delvenne P . Colonization of in vitro-formed cervical human papillomavirus- associated (pre)neoplastic lesions with dendritic cells: role of granulocyte/macrophage colony-stimulating factor. Am J Pathol 1999; 154: 775–784.
Merrick DT, Blanton RA, Gown AM, McDougall JK . Altered expression of proliferation and differentiation markers in human papillomavirus 16 and 18 immortalized epithelial cells grown in organotypic culture. Am J Pathol 1992; 140: 167–177.
Deroo S, Defrancq E, Moucheron C, Kirsch-De Mesmaeker A, Dumy P . Synthesis of an oxyamino-containing phenanthroline derivative for the efficient preparation of phenanthroline oligonucleotide oxime conjugates. Tetrahedron Letters 2003; 44: 8379–8382.
Deroo S, Le Gac S, Ghosh S, Villien M, Gerbaux P, Defrancq E et al. Oligonucleotide duplexes with tethered photoreactive ruthenium(II) complexes: influence of the ligands and their linker on the photoinduced electron transfer and crosslinking processes of the two strands. Eur J Inorg Chem 2009: 524–532.
Villien M, Deroo S, Gicquel E, Defrancq E, Moucheron C, Kirsch-De Mesmaeker A . The oxime bond formation as an efficient tool for the conjugation of ruthenium complexes to oligonucleotides and peptides. Tetrahedron 2007; 63: 11299–11306.
Acknowledgements
The research presented here was supported by the Walloon Region (CARCINOM Waleo-2 project). LM thanks the FNRS for a fellowship (‘aspirant’ at the FNRS). The authors are grateful to a NanoBio programme (Grenoble) for the facilities of the Synthesis platform.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Rights and permissions
About this article
Cite this article
Reschner, A., Bontems, S., Le Gac, S. et al. Ruthenium oligonucleotides, targeting HPV16 E6 oncogene, inhibit the growth of cervical cancer cells under illumination by a mechanism involving p53. Gene Ther 20, 435–443 (2013). https://doi.org/10.1038/gt.2012.54
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/gt.2012.54
Keywords
This article is cited by
-
Human papillomavirus infection, cervical cancer and the less explored role of trace elements
Biological Trace Element Research (2023)