Abstract
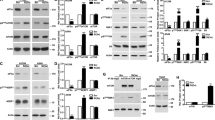
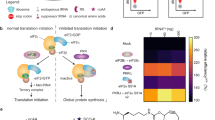
Nucleofection permits efficient transfection even with difficult cell types such as primary and non-dividing cells, and is used to deliver various nucleic acids, including DNA, mRNA, and small interfering RNA. Unlike DNA and small interfering RNA, mRNA is subject to rapid degradation, which necessitates instant early translation following mRNA delivery. We examined the factors that are important in translation following nucleofection and observed rapid phosphorylation of eukaryotic initiation factor 2 alpha (eIF2α) following nucleofection, which occurred in the absence of the delivered nucleic acid. We studied the involvement of three ubiquitous kinases capable of phosphorylating eIF2α in mammalian cells and identified that nucleofection-mediated phosphorylation of eIF2α was dependent on general control non-derepressible 2 (GCN2) and RNA-dependent protein kinase (PKR)-like endoplasmic reticulum kinase (PERK) but not PKR. A reduction in translation due to eIF2α phosphorylation was observed post nucleofection, demonstrating functional significance. Understanding the impact of nucleofection on translational machinery has important implications for therapeutics currently under development based on the delivery of mRNA, DNA, and small interfering RNA. Strategies to circumvent eIF2α phosphorylation and other downstream effects of activating GCN2 and PERK will facilitate further advancement of nucleic acid-based therapies.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
References
Gresch O, Engel FB, Nesic D, Tran TT, England HM, Hickman ES et al. New non-viral method for gene transfer into primary cells. Methods 2004; 33: 151–163.
Hamm A, Krott N, Breibach I, Blindt R, Bosserhoff AK . Efficient transfection method for primary cells. Tissue Eng 2002; 8: 235–245.
Harriague J, Bismuth G . Imaging antigen-induced PI3K activation in T cells. Nat Immunol 2002; 3: 1090–1096.
Coughlin CM, Vance BA, Grupp SA, Vonderheide RH . RNA-transfected CD40-activated B cells induce functional T-cell responses against viral and tumor antigen targets: implications for pediatric immunotherapy. Blood 2004; 103: 2046–2054.
Van De Parre TJ, Martinet W, Schrijvers DM, Herman AG, De Meyer GR . mRNA but not plasmid DNA is efficiently transfected in murine J774A.1 macrophages. Biochem Biophys Res Commun 2005; 327: 356–360.
Schacht V, Ramirez MI, Hong YK, Hirakawa S, Feng D, Harvey N et al. T1alpha/podoplanin deficiency disrupts normal lymphatic vasculature formation and causes lymphedema. EMBO J 2003; 22: 3546–3556.
Poy MN, Eliasson L, Krutzfeldt J, Kuwajima S, Ma X, Macdonald PE et al. A pancreatic islet-specific microRNA regulates insulin secretion. Nature 2004; 432: 226–230.
Vo N, Klein ME, Varlamova O, Keller DM, Yamamoto T, Goodman RH et al. A cAMP-response element binding protein-induced microRNA regulates neuronal morphogenesis. Proc Natl Acad Sci USA 2005; 102: 16426–16431.
Roy J, Paquette JS, Fortin JF, Tremblay MJ . The immunosuppressant rapamycin represses human immunodeficiency virus type 1 replication. Antimicrob Agents Chemother 2002; 46: 3447–3455.
Blindt R, Zeiffer U, Krott N, Filzmaier K, Voss M, Hanrath P et al. Upregulation of the cytoskeletal-associated protein Moesin in the neointima of coronary arteries after balloon angioplasty: a new marker of smooth muscle cell migration? Cardiovasc Res 2002; 54: 630–639.
Hacein-Bey-Abina S, Von Kalle C, Schmidt M, McCormack MP, Wulffraat N, Leboulch P et al. LMO2-associated clonal T cell proliferation in two patients after gene therapy for SCID-X1. Science 2003; 302: 415–419.
Weissman D, Ni H, Scales D, Dude A, Capodici J, McGibney K et al. HIV gag mRNA transfection of dendritic cells (DC) delivers encoded antigen to MHC class I and II molecules, causes DC maturation, and induces a potent human in vitro primary immune response. J Immunol 2000; 165: 4710–4717.
Cannon G, Weissman D . RNA based vaccines. DNA Cell Biol 2002; 21: 953–961.
Zhao Y, Moon E, Carpenito C, Paulos CM, Liu X, Brennan AL et al. Multiple injections of electroporated autologous T cells expressing a chimeric antigen receptor mediate regression of human disseminated tumor. Cancer Res 2010; 70: 9053–9061.
Holcik M, Sonenberg N . Translational control in stress and apoptosis. Nat Rev Mol Cell Biol 2005; 6: 318–327.
Nallagatla SR, Toroney R, Bevilacqua PC . Regulation of innate immunity through RNA structure and the protein kinase PKR. Curr Opin Struct Biol 2011; 21: 119–127.
Daher A, Laraki G, Singh M, Melendez-Pena CE, Bannwarth S, Peters AH et al. TRBP control of PACT-induced phosphorylation of protein kinase R is reversed by stress. Mol Cell Biol 2009; 29: 254–265.
Pindel A, Sadler A . The role of protein kinase R in the interferon response. J Interferon Cytokine Res 2011; 31: 59–70.
Lu L, Han AP, Chen JJ . Translation initiation control by heme-regulated eukaryotic initiation factor 2alpha kinase in erythroid cells under cytoplasmic stresses. Mol Cell Biol 2001; 21: 7971–7980.
Bertolotti A, Zhang Y, Hendershot LM, Harding HP, Ron D . Dynamic interaction of BiP and ER stress transducers in the unfolded-protein response. Nat Cell Biol 2000; 2: 326–332.
Harding HP, Novoa I, Zhang Y, Zeng H, Wek R, Schapira M et al. Regulated translation initiation controls stress-induced gene expression in mammalian cells. Mol Cell 2000; 6: 1099–1108.
Wek SA, Zhu S, Wek RC . The histidyl-tRNA synthetase-related sequence in the eIF-2 alpha protein kinase GCN2 interacts with tRNA and is required for activation in response to starvation for different amino acids. Mol Cell Biol 1995; 15: 4497–4506.
Jiang HY, Wek RC . Phosphorylation of the alpha-subunit of the eukaryotic initiation factor-2 (eIF2alpha) reduces protein synthesis and enhances apoptosis in response to proteasome inhibition. J Biol Chem 2005; 280: 14189–14202.
Deng J, Harding HP, Raught B, Gingras AC, Berlanga JJ, Scheuner D et al. Activation of GCN2 in UV-irradiated cells inhibits translation. Curr Biol 2002; 12: 1279–1286.
Anderson BR, Muramatsu H, Nallagatla SR, Bevilacqua PC, Sansing LH, Weissman D et al. Incorporation of pseudouridine into mRNA enhances translation by diminishing PKR activation. Nucleic Acids Res 2010; 38: 5884–5892.
Kariko K, Muramatsu H, Ludwig J, Weissman D . Generating the optimal mRNA for therapy: HPLC purification eliminates immune activation and improves translation of nucleoside-modified, protein-encoding mRNA. Nucleic Acids Res 2011; 39: e142.
Underhill MF, Coley C, Birch JR, Findlay A, Kallmeier R, Proud CG et al. Engineering mRNA translation initiation to enhance transient gene expression in Chinese hamster ovary cells. Biotechnol Prog 2003; 19: 121–129.
Tesfay MZ, Yin J, Gardner CL, Khoretonenko MV, Korneeva NL, Rhoads RE et al. Alpha/beta interferon inhibits cap-dependent translation of viral but not cellular mRNA by a PKR-independent mechanism. J Virol 2008; 82: 2620–2630.
Wu S, Hu Y, Wang JL, Chatterjee M, Shi Y, Kaufman RJ . Ultraviolet light inhibits translation through activation of the unfolded protein response kinase PERK in the lumen of the endoplasmic reticulum. J Biol Chem 2002; 277: 18077–18083.
Dever TE, Dar AC, Sicheri F . The eIF2alpha kinases. In: Mathews MB, Sonenberg N, Hershey JWB (eds). Translational Control in Biology and Medicine. Cold Spring Harbor Laboratory Press: Cold Spring Harbor, NY, 2007, pp 319–344.
Hinnebusch AG . Translational regulation of GCN4 and the general amino acid control of yeast. Annu Rev Microbiol 2005; 59: 407–450.
Shurin MR, Gregory M, Morris JC, Malyguine AM . Genetically modified dendritic cells in cancer immunotherapy: a better tomorrow? Expert Opin Biol Ther 2010; 10: 1539–1553.
Gilboa E, Vieweg J . Cancer immunotherapy with mRNA-transfected dendritic cells. Immunol Rev 2004; 199: 251–263.
Lesterhuis WJ, De Vries IJ, Schreibelt G, Schuurhuis DH, Aarntzen EH, De Boer A et al. Immunogenicity of dendritic cells pulsed with CEA peptide or transfected with CEA mRNA for vaccination of colorectal cancer patients. Anticancer Res 2010; 30: 5091–5097.
Perche F, Benvegnu T, Berchel M, Lebegue L, Pichon C, Jaffres PA et al. Enhancement of dendritic cells transfection in vivo and of vaccination against B16F10 melanoma with mannosylated histidylated lipopolyplexes loaded with tumor antigen messenger RNA. Nanomedicine 2011; 7: 445–453.
Van Tendeloo VF, Van de Velde A, Van Driessche A, Cools N, Anguille S, Ladell K et al. Induction of complete and molecular remissions in acute myeloid leukemia by Wilms’ tumor 1 antigen-targeted dendritic cell vaccination. Proc Natl Acad Sci USA 2010; 107: 13824–13829.
Pascolo S . Vaccination with messenger RNA (mRNA). Handb Exp Pharmacol 2008; 183: 221–235.
Birkholz K, Hombach A, Krug C, Reuter S, Kershaw M, Kampgen E et al. Transfer of mRNA encoding recombinant immunoreceptors reprograms CD4+ and CD8+ T cells for use in the adoptive immunotherapy of cancer. Gene Therapy 2009; 16: 596–604.
Ponsaerts P, van der Sar S, Van Tendeloo VF, Jorens PG, Berneman ZN, Singh PB . Highly efficient mRNA-based gene transfer in feeder-free cultured H9 human embryonic stem cells. Cloning Stem Cells 2004; 6: 211–216.
Angel M, Yanik MF . Innate immune suppression enables frequent transfection with RNA encoding reprogramming proteins. PLoS One 2010; 5: e11756.
Kaji K, Norrby K, Paca A, Mileikovsky M, Mohseni P, Woltjen K . Virus-free induction of pluripotency and subsequent excision of reprogramming factors. Nature 2009; 458: 771–775.
Sul JY, Wu CW, Zeng F, Jochems J, Lee MT, Kim TK et al. Transcriptome transfer produces a predictable cellular phenotype. Proc Natl Acad Sci USA 2009; 106: 7624–7629.
Warren L, Manos PD, Ahfeldt T, Loh Y-H, Li H, Lau F et al. Highly efficient reprogramming to pluripotency and directed differentiation of human cells with synthetic modified mRNA. Cell Stem Cell 2010; 7: 618–630.
Yakubov E, Rechavi G, Rozenblatt S, Givol D . Reprogramming of human fibroblasts to pluripotent stem cells using mRNA of four transcription factors. Biochem Biophys Res Commun 2010; 394: 189–193.
Im GI, Kim HJ . Electroporation-mediated gene transfer of SOX trio to enhance chondrogenesis in adipose stem cells. Osteoarthritis Cartilage 2011; 19: 449–457.
Mallilankaraman K, Shedlock DJ, Bao H, Kawalekar OU, Fagone P, Ramanathan AA et al. A DNA vaccine against chikungunya virus is protective in mice and induces neutralizing antibodies in mice and nonhuman primates. PLoS Negl Trop Dis 2011; 5: e928.
Scheuner D, Song B, McEwen E, Liu C, Laybutt R, Gillespie P et al. Translational control is required for the unfolded protein response and in vivo glucose homeostasis. Mol Cell 2001; 7: 1165–1176.
Zeenko VV, Wang C, Majumder M, Komar AA, Snider MD, Merrick WC et al. An efficient in vitro translation system from mammalian cells lacking the translational inhibition caused by eIF2 phosphorylation. RNA 2008; 14: 593–602.
Duncan R, Hershey JW . Regulation of initiation factors during translational repression caused by serum depletion. Abundance, synthesis, and turnover rates. J Biol Chem 1985; 260: 5486–5492.
Jousse C, Oyadomari S, Novoa I, Lu P, Zhang Y, Harding HP et al. Inhibition of a constitutive translation initiation factor 2alpha phosphatase, CReP, promotes survival of stressed cells. J Cell Biol 2003; 163: 767–775.
Kawagishi-Kobayashi M, Silverman JB, Ung TL, Dever TE . Regulation of the protein kinase PKR by the vaccinia virus pseudosubstrate inhibitor K3L is dependent on residues conserved between the K3L protein and the PKR substrate eIF2alpha. Mol Cell Biol 1997; 17: 4146–4158.
Kariko K, Buckstein M, Ni H, Weissman D . Suppression of RNA recognition by Toll-like receptors: the impact of nucleoside modification and the evolutionary origin of RNA. Immunity 2005; 23: 165–175.
Kariko K, Muramatsu H, Welsh FA, Ludwig J, Kato H, Akira S et al. Incorporation of pseudouridine into mRNA yields superior nonimmunogenic vector with increased translational capacity and biological stability. Mol Ther 2008; 16: 1833–1840.
Acknowledgements
MEF cell lines were generously provided by David Ron (WT, GCN2−/−, and PERK−/−), Robert Silverman (PKR−/−) and Alan Diehl (with permission from Douglas Cavener) (PERK−/−/GCN2−/−). This work was supported by the National Institutes of Health (R01AI50484 and R21DE019059 to DW, T32DK07748 to BRA, R42HL87688 to KK).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Rights and permissions
About this article
Cite this article
Anderson, B., Karikó, K. & Weissman, D. Nucleofection induces transient eIF2α phosphorylation by GCN2 and PERK. Gene Ther 20, 136–142 (2013). https://doi.org/10.1038/gt.2012.5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/gt.2012.5
Keywords
This article is cited by
-
The cellular response to plasma membrane disruption for nanomaterial delivery
Nano Convergence (2022)
-
Intracellular Delivery of mRNA in Adherent and Suspension Cells by Vapor Nanobubble Photoporation
Nano-Micro Letters (2020)
-
A researcher’s guide to the galaxy of IRESs
Cellular and Molecular Life Sciences (2017)