Abstract
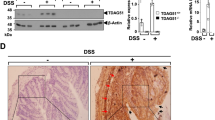
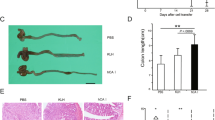
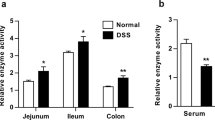
Cathelicidin is a pleiotropic host defense peptide secreted by epithelial and immune cells. Whether endogenous cathelicidin is protective against ulcerative colitis, however, is unclear. Here we sought to delineate the role of endogenous murine cathelicidin (mCRAMP) and the therapeutic efficacy of intrarectal administration of mCRAMP-encoding plasmid in ulcerative colitis using dextran sulfate sodium (DSS)-challenged cathelicidin-knockout (Cnlp−/−) mice as a model. Cnlp−/− mice had more severe symptoms and mucosal disruption than the wild-type mice in response to DSS challenge. The tissue levels of interleukin-1β and tumor necrosis factor-α, myeloperoxidase activity and the number of apoptotic cells were increased in the colon of DSS-challenged Cnlp−/− mice. Moreover, mucus secretion and mucin gene expression were impaired in Cnlp−/− mice. All these abnormalities were reversed by the intrarectal administration of mCRAMP or mCRAMP-encoding plasmid. Taken together, endogenous cathelicidin may protect against ulcerative colitis through modulation of inflammation and mucus secretion.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Khor B, Gardet A, Xavier RJ . Genetics and pathogenesis of inflammatory bowel disease. Nature 2011; 454: 307–317.
Maloy KJ, Powrie F . Intestinal homeostasis and its breakdown in inflammatory bowel disease. Nature 2011; 474: 298–306.
Gibson PR, Muir JG . Reinforcing the mucus: a new therapeutic approach for ulcerative colitis? Gut 2005; 54: 900–903.
Pullan RD, Thomas GA, Rhodes M, Newcombe RG, Williams GT, Allen A et al. Thickness of adherent mucus gel on colonic mucosa in humans and its relevance to colitis. Gut 1994; 35: 353–359.
Van der Sluis M, De Koning BA, De Bruijn AC, Velcich A, Meijerink JP, Van Goudoever JB et al. Muc2-deficient mice spontaneously develop colitis, indicating that MUC2 is critical for colonic protection. Gastroenterology 2006; 131: 117–129.
van Dullemen HM, van Deventer SJ, Hommes DW, Bijl HA, Jansen J, Tytgat GN et al. Treatment of Crohn's disease with anti-tumor necrosis factor chimeric monoclonal antibody (cA2). Gastroenterology 1995; 109: 129–135.
Sasaki M, Mathis JM, Jennings MH, Jordan P, Wang Y, Ando T et al. Reversal of experimental colitis disease activity in mice following administration of an adenoviral IL-10 vector. J Inflamm (Lond) 2005; 2: 13.
Domenech E . Inflammatory bowel disease: current therapeutic options. Digestion 2006; 73 (Suppl 1): 67–76.
Ullman TA, Itzkowitz SH . Intestinal inflammation and cancer. Gastroenterology 2011; 140: 1807–1816.
Hase K, Eckmann L, Leopard JD, Varki N, Kagnoff MF . Cell differentiation is a key determinant of cathelicidin LL-37/human cationic antimicrobial protein 18 expression by human colon epithelium. Infect Immun 2002; 70: 953–963.
Metz-Boutigue MH, Shooshtarizadeh P, Prevost G, Haikel Y, Chich JF . Antimicrobial peptides present in mammalian skin and gut are multifunctional defence molecules. Curr Pharm Des 2010; 16: 1024–1039.
De Y, Chen Q, Schmidt AP, Anderson GM, Wang JM, Wooters J et al. LL-37, the neutrophil granule- and epithelial cell-derived cathelicidin, utilizes formyl peptide receptor-like 1 (FPRL1) as a receptor to chemoattract human peripheral blood neutrophils, monocytes, and T cells. J Exp Med 2000; 192: 1069–1074.
Wu WK, Wong CC, Li ZJ, Zhang L, Ren SX, Cho CH . Cathelicidins in inflammation and tissue repair: potential therapeutic applications for gastrointestinal disorders. Acta Pharmacol Sin 2010; 31: 1118–1122.
Rosenfeld Y, Papo N, Shai Y . Endotoxin (lipopolysaccharide) neutralization by innate immunity host-defense peptides. Peptide properties and plausible modes of action. J Biol Chem 2006; 281: 1636–1643.
Tahara Y, Ido A, Yamamoto S, Miyata Y, Uto H, Hori T et al. Hepatocyte growth factor facilitates colonic mucosal repair in experimental ulcerative colitis in rats. J Pharmacol Exp Ther 2003; 307: 146–151.
Tai EK, Wu WK, Wong HP, Lam EK, Yu L, Cho CH . A new role for cathelicidin in ulcerative colitis in mice. Exp Biol Med (Maywood) 2007; 232: 799–808.
Koon HW, Shih DQ, Chen J, Bakirtzi K, Hing TC, Law I et al. Cathelicidin signaling via the toll-like receptor protects against colitis in mice. Gastroenterology 2011; 141: 1852–1863. e3.
Schauber J, Rieger D, Weiler F, Wehkamp J, Eck M, Fellermann K et al. Heterogeneous expression of human cathelicidin hCAP18/LL-37 in inflammatory bowel diseases. Eur J Gastroenterol Hepatol 2006; 18: 615–621.
Kanbe T, Murai R, Mukoyama T, Murawaki Y, Hashiguchi K, Yoshida Y et al. Naked gene therapy of hepatocyte growth factor for dextran sulfate sodium-induced colitis in mice. Biochem Biophys Res Commun 2006; 345: 1517–1525.
Mankertz J, Schulzke JD . Altered permeability in inflammatory bowel disease: pathophysiology and clinical implications. Curr Opin Gastroenterol 2007; 23: 379–383.
Corfield AP, Wagner SA, Clamp JR, Kriaris MS, Hoskins LC . Mucin degradation in the human colon: production of sialidase, sialate O-acetylesterase, N-acetylneuraminate lyase, arylesterase, and glycosulfatase activities by strains of fecal bacteria. Infect Immun 1992; 60: 3971–3978.
Pullman WE, Elsbury S, Kobayashi M, Hapel AJ, Doe WF . Enhanced mucosal cytokine production in inflammatory bowel disease. Gastroenterology 1992; 102: 529–537.
Strober W, Fuss IJ . Proinflammatory cytokines in the pathogenesis of inflammatory bowel diseases. Gastroenterology 2011; 140: 1756–1767.
Stevens C, Walz G, Singaram C, Lipman ML, Zanker B, Muggia A et al. Tumor necrosis factor-alpha, interleukin-1 beta, and interleukin-6 expression in inflammatory bowel disease. Dig Dis Sci 1992; 37: 818–826.
Bouguen G, Chevaux JB, Peyrin-Biroulet L . Recent advances in cytokines: therapeutic implications for inflammatory bowel diseases. World J Gastroenterol 2011; 17: 547–556.
Nizet V, Ohtake T, Lauth X, Trowbridge J, Rudisill J, Dorschner RA et al. Innate antimicrobial peptide protects the skin from invasive bacterial infection. Nature 2001; 414: 454–457.
Liu ES, Ye YN, Shin VY, Yuen ST, Leung SY, Wong BC et al. Cigarette smoke exposure increases ulcerative colitis-associated colonic adenoma formation in mice. Carcinogenesis 2003; 24: 1407–1413.
Cooper HS, Murthy SN, Shah RS, Sedergran DJ . Clinicopathologic study of dextran sulfate sodium experimental murine colitis. Lab Invest 1993; 69: 238–249.
Guo X, Wang WP, Ko JK, Cho CH . Involvement of neutrophils and free radicals in the potentiating effects of passive cigarette smoking on inflammatory bowel disease in rats. Gastroenterology 1999; 117: 884–892.
Ma L, Wang WP, Chow JY, Lam SK, Cho CH . The role of polyamines in gastric mucus synthesis inhibited by cigarette smoke or its extract. Gut 2000; 47: 170–177.
Gavrieli Y, Sherman Y, Ben-Sasson SA . Identification of programmed cell death in situ via specific labeling of nuclear DNA fragmentation. J Cell Biol 1992; 119: 493–501.
Acknowledgements
This work is partially supported by the Hong Kong Research Grants Council (CUHK 7565/06M).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Rights and permissions
About this article
Cite this article
Tai, E., Wu, W., Wang, X. et al. Intrarectal administration of mCRAMP-encoding plasmid reverses exacerbated colitis in Cnlp−/− mice. Gene Ther 20, 187–193 (2013). https://doi.org/10.1038/gt.2012.22
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/gt.2012.22
Keywords
This article is cited by
-
Anti-inflammatory and antibacterial effects of human cathelicidin active fragment KR-12 in the mouse models of colitis: a novel potential therapy of inflammatory bowel diseases
Pharmacological Reports (2021)
-
Antimicrobial host defence peptides: functions and clinical potential
Nature Reviews Drug Discovery (2020)
-
High salt diet exacerbates colitis in mice by decreasing Lactobacillus levels and butyrate production
Microbiome (2018)
-
High therapeutic efficacy of Cathelicidin-WA against postweaning diarrhea via inhibiting inflammation and enhancing epithelial barrier in the intestine
Scientific Reports (2016)
-
Colonic MUC2 mucin regulates the expression and antimicrobial activity of β-defensin 2
Mucosal Immunology (2015)