Abstract
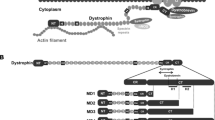
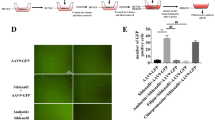
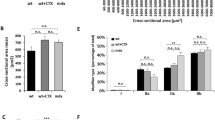
Duchenne muscular dystrophy (DMD) is the most common form of the progressive muscular dystrophies characterized by defects of the dystrophin gene. Although primarily characterized by degeneration of the limb muscles, cardiomyopathy is a major cause of death. Therefore, the development of curative modalities such as gene therapy is imperative. We evaluated the cardiomyopathic features of mdx mice to observe improvements in response to intravenous administration of recombinant adeno-associated virus (AAV) type 9 encoding microdystrophin. The myocardium was extensively transduced with microdystrophin to significantly prevent the development of fibrosis, and expression persisted for the duration of the study. Intraventricular conduction patterns, such as the QRS complex duration and S/R ratio in electrocardiography, were also corrected, indicating that the transduced microdystrophin has a protective effect on the dystrophin-deficient myocardium. Furthermore, BNP and ANP levels were reduced to normal, suggesting the absence of cardiac dysfunction. In aged mice, prevention of ectopic beats as well as echocardiographic amelioration was also demonstrated with improved exercise performance. These findings indicate that AAV-mediated cardiac transduction with microdystrophin might be a promising therapeutic strategy for the treatment of dystrophin-deficient cardiomyopathy.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout








Similar content being viewed by others
References
Emery AE . Population frequencies of inherited neuromuscular diseases--a world survey. Neuromuscul Disord 1991; 1: 19–29.
Aartsma-Rus A, Van Deutekom JCT, Fokkema IF, Van Ommen G-JB, Den Dunnen JT . Entries in the Leiden Duchenne muscular dystrophy mutation database: an overview of mutation types and paradoxical cases that confirm the reading-frame rule. Muscle Nerve 2006; 34: 135–144.
Bushby K, Muntoni F, Bourke JP . 107th ENMC international workshop: the management of cardiac involvement in muscular dystrophy and myotonic dystrophy. 7th–9th June 2002, Naarden, the Netherlands. Neuromuscul Disord 2003; 13: 166–172.
Moxley RT . Clinical overview of Duchenne muscular dystrophy. In: Chamberlain J, Rando T (eds). Duchenne Muscular Dystrophy: Advances in Therapeutics. Taylor & Francis Group: New York, 2006, pp 1–20.
Nigro G, Comi LI, Politano L, Nigro G . Cardiomyopathies associated with muscular dystrophies. In: Engel A, Franzini-Armstrong C (eds). Myology: Basic and Clinical. McGraw-Hill: New York, 2004, pp 1239–1256.
Connuck DM, Sleeper LA, Colan SD, Cox GF, Towbin JA, Lowe AM et al. Characteristics and outcomes of cardiomyopathy in children with Duchenne or Becker muscular dystrophy: a comparative study from the Pediatric Cardiomyopathy Registry. Am Heart J 2008; 155: 998–1005.
Gray SJ, Samulski RJ . Optimizing gene delivery vectors for the treatment of heart disease. Expert Opin Biol Ther 2008; 8: 911–922.
Hoffman EP, Brown RH, Kunkel LM . Dystrophin: the protein product of the Duchenne muscular dystrophy locus. Cell 1987; 51: 919–928.
Dong JY, Fan PD, Frizzell RA . Quantitative analysis of the packaging capacity of recombinant adeno-associated virus. Hum Gene Therapy 1996; 7: 2101–2112.
Yuasa K, Miyagoe Y, Yamamoto K, Nabeshima Y, Dickson G, Takeda S . Effective restoration of dystrophin-associated proteins in vivo by adenovirus-mediated transfer of truncated dystrophin cDNAs. FEBS Lett 1998; 425: 329–336.
Sakamoto M, Yuasa K, Yoshimura M, Yokota T, Ikemoto T, Suzuki M et al. Micro-dystrophin cDNA ameliorates dystrophic phenotypes when introduced into mdx mice as a transgene. Biochem Biophys Res Commun 2002; 293: 1265–1272.
Yoshimura M, Sakamoto M, Ikemoto M, Mochizuki Y, Yuasa K, Miyagoe-Suzuki Y et al. AAV vector-mediated microdystrophin expression in a relatively small percentage of mdx myofibers improved the mdx phenotype. Mol Ther 2004; 10: 821–828.
Gregorevic P, Allen JM, Minami E, Blankinship MJ, Haraguchi M, Meuse L et al. rAAV6-microdystrophin preserves muscle function and extends lifespan in severely dystrophic mice. Nat Med 2006; 12: 787–789.
Ohshima S, Shin JH, Yuasa K, Nishiyama A, Kira J, Okada T et al. Transduction efficiency and immune response associated with the administration of AAV8 vector into dog skeletal muscle. Mol Ther 2009; 17: 73–80.
Inagaki K, Fuess S, Storm TA, Gibson GA, McTiernan CF, Kay MA et al. Robust systemic transduction with AAV9 vectors in mice: efficient global cardiac gene transfer superior to that of AAV8. Mol Ther 2006; 14: 45–53.
Bulfield G, Siller WG, Wight PA, Moore KJ . X chromosome-linked muscular dystrophy (mdx) in the mouse. Proc Natl Acad Sci USA 1984; 81: 1189–1192.
Sicinski P, Geng Y, Ryder-Cook AS, Barnard EA, Darlison MG, Barnard PJ . The molecular basis of muscular dystrophy in the mdx mouse: a point mutation. Science 1989; 244: 1578–1580.
Im WB, Phelps SF, Copen EH, Adams EG, Slightom JL, Chamberlain JS . Differential expression of dystrophin isoforms in strains of mdx mice with different mutations. Hum Mol Genet 1996; 5: 1149–1153.
Sapp JL, Bobet J, Howlett SE . Contractile properties of myocardium are altered in dystrophin-deficient mdx mice. J Neurol Sci 1996; 142: 17–24.
Bridges LR . The association of cardiac muscle necrosis and inflammation with the degenerative and persistent myopathy of MDX mice. J Neurol Sci 1986; 72: 147–157.
Bostick B, Yue Y, Lai Y, Long C, Li D, Duan D . Adeno-associated virus serotype-9 microdystrophin gene therapy ameliorates electrocardiographic abnormalities in mdx mice. Hum Gene Therapy 2008; 19: 851–856.
Townsend D, Blankinship MJ, Allen JM, Gregorevic P, Chamberlain JS, Metzger JM . Systemic administration of micro-dystrophin restores cardiac geometry and prevents dobutamine-induced cardiac pump failure. Mol Ther 2007; 15: 1086–1092.
Chu V, Otero JM, Lopez O, Sullivan MF, Morgan JP, Amende I et al. Electrocardiographic findings in mdx mice: a cardiac phenotype of Duchenne muscular dystrophy. Muscle Nerve 2002; 26: 513–519.
Pacak CA, Mah CS, Thattaliyath BD, Conlon TJ, Lewis MA, Cloutier DE et al. Recombinant adeno-associated virus serotype 9 leads to preferential cardiac transduction in vivo. Circ Res 2006; 99: e3–e9.
Janssen PML, Hiranandani N, Mays TA, Rafael-Fortney JA . Utrophin deficiency worsens cardiac contractile dysfunction present in dystrophin-deficient mdx mice. Am J Physiol Heart Circ Physiol 2005; 289: H2373–H2378.
Quinlan JG, Hahn HS, Wong BL, Lorenz JN, Wenisch AS, Levin LS . Evolution of the mdx mouse cardiomyopathy: physiological and morphological findings. Neuromuscul Disord 2004; 14: 491–496.
Yue Y, Skimming JW, Liu M, Strawn T, Duan D . Full-length dystrophin expression in half of the heart cells ameliorates beta-isoproterenol-induced cardiomyopathy in mdx mice. Hum Mol Genet 2004; 13: 1669–1675.
Mann DL . Heart failure and cor pulmonale. In: Fauci AS, Braunwald E, Kasper DL et al. (eds) Harrison's Principles of Internal Medicine. 17th edn. chapter 227. McGraw Hill: New York, 2008, pp 1443–1457.
Dunlap ME, Bibevski S, Rosenberry TL, Ernsberger P . Mechanisms of altered vagal control in heart failure: influence of muscarinic receptors and acetylcholinesterase activity. Am J Physiol Heart Circ Physiol 2003; 285: H1632–H1640.
Das G, Talmers FN, Weissler AM . New observations on the effects of atropine on the sinoatrial and atrioventricular nodes in man. Am J Cardiol 1975; 36: 281–285.
Benditt DG, Pritchett LC, Smith WM, Wallace AG, Gallagher JJ . Characteristics of atrioventricular conduction and the spectrum of arrhythmias in Lown-Ganong-Levine syndrome. Circulation 1978; 57: 454–465.
Ansong AK, Li JS, Nozik-Grayck E, Ing R, Kravitz RM, Idriss SF et al. Electrocardiographic response to enzyme replacement therapy for Pompe disease. Genet Med 2006; 8: 297–301.
Linhart A, Lubanda JC, Palecek T, Bultas J, Karetová D, Ledvinová J et al. Cardiac manifestations in Fabry disease. J Inherit Metab Dis 2001; 24 (Suppl 2): 75–83.
Kashani A, Barold SS . Significance of QRS complex duration in patients with heart failure. J Am Coll Cardiol 2005; 46: 2183–2192.
Bia BL, Cassidy PJ, Young ME, Rafael JA, Leighton B, Davies KE et al. Decreased myocardial nNOS, increased iNOS and abnormal ECGs in mouse models of Duchenne muscular dystrophy. J Mol Cell Cardiol 1999; 31: 1857–1862.
Frankel KA, Rosser RJ . The pathology of the heart in progressive muscular dystrophy: epimyocardial fibrosis. Hum Pathol 1976; 7: 375–386.
Yuasa K, Sakamoto M, Miyagoe-Suzuki Y, Tanouchi A, Yamamoto H, Li J et al. Adeno-associated virus vector-mediated gene transfer into dystrophin-deficient skeletal muscles evokes enhanced immune response against the transgene product. Gene Therapy 2002; 9: 1576–1588.
Lin J, Zhi Y, Mays L, Wilson JM . Vaccines based on novel adeno-associated virus vectors elicit aberrant CD8+ T-cell responses in mice. J Virol 2007; 81: 11840–11849.
Matsushita T, Elliger S, Elliger C, Podsakoff G, Villarreal L, Kurtzman GJ et al. Adeno-associated virus vectors can be efficiently produced without helper virus. Gene Therapy 1998; 5: 938–945.
Okada T, Nomoto T, Yoshioka T, Nonaka-Sarukawa M, Ito T, Ogura T et al. Large-scale production of recombinant viruses by use of a large culture vessel with active gassing. Hum Gene Ther 2005; 16: 1212–1218.
Okada T, Nonaka-Sarukawa M, Uchibori R, Kinoshita K, Hayashita-Kinoh H, Nitahara-Kasahara Y et al. Scalable purification of adeno-associated virus serotype 1 (AAV1) and AAV8 vectors, using dual ion-exchange adsorptive membranes. Hum Gene Ther 2009; 20: 1013–1021.
Pruitt KD, Tatusova T, Klimke W, Maglott DR . NCBI reference sequences: current status, policy and new initiatives. Nucleic Acids Res 2009; 37: D32–D36.
Fayssoil A . Tissue Doppler characterization of cardiac phenotype in mouse. Eur J Radiol 2009; 72: 82–84.
Acknowledgements
We thank Dr James M Wilson for providing a helper plasmid pAAV2/9. This work was supported by a Grant for Research on Nervous and Mental Disorders, by Health Science Research Grants for Research on the Human Genome and Gene Therapy, by Research on Brain Science from the Ministry of Health, Labor, and Welfare, and by Grants in Aid for Scientific Research from the Ministry of Education, Culture, Sports, Science, and Technology.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Supplementary Information accompanies the paper on Gene Therapy website
Rights and permissions
About this article
Cite this article
Shin, JH., Nitahara-Kasahara, Y., Hayashita-Kinoh, H. et al. Improvement of cardiac fibrosis in dystrophic mice by rAAV9-mediated microdystrophin transduction. Gene Ther 18, 910–919 (2011). https://doi.org/10.1038/gt.2011.36
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/gt.2011.36
Keywords
This article is cited by
-
CDP-ribitol prodrug treatment ameliorates ISPD-deficient muscular dystrophy mouse model
Nature Communications (2022)
-
Long-Term Protective Effect of Human Dystrophin Expressing Chimeric (DEC) Cell Therapy on Amelioration of Function of Cardiac, Respiratory and Skeletal Muscles in Duchenne Muscular Dystrophy
Stem Cell Reviews and Reports (2022)
-
Evaluation of the dystrophin carboxy-terminal domain for micro-dystrophin gene therapy in cardiac and skeletal muscles in the DMDmdx rat model
Gene Therapy (2022)
-
Low human dystrophin levels prevent cardiac electrophysiological and structural remodelling in a Duchenne mouse model
Scientific Reports (2021)
-
100-fold but not 50-fold dystrophin overexpression aggravates electrocardiographic defects in the mdx model of Duchenne muscular dystrophy
Molecular Therapy - Methods & Clinical Development (2016)