Abstract
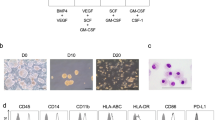
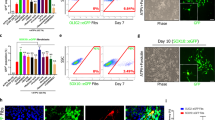
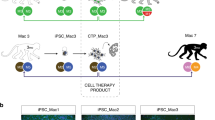
This report describes generation of dendritic cells (DCs) and macrophages from human induced pluripotent stem (iPS) cells. iPS cell-derived DC (iPS-DC) exhibited the morphology of typical DC and function of T-cell stimulation and antigen presentation. iPS-DC loaded with cytomegalovirus (CMV) peptide induced vigorous expansion of CMV-specific autologous CD8+ T cells. Macrophages (iPS-MP) with activity of zymosan phagocytosis and C5a-induced chemotaxis were also generated from iPS cells. Genetically modified iPS-MPs were generated by the introduction of expression vectors into undifferentiated iPS cells, isolation of transfectant iPS cell clone and subsequent differentiation. By this procedure, we generated iPS-MP expressing a membrane-bound form of single chain antibody (scFv) specific to amyloid β (Aβ), the causal protein of Alzheimer’s disease. The scFv-transfectant iPS-MP exhibited efficient Aβ-specific phagocytosis activity. iPS-MP expressing CD20-specific scFv engulfed and killed BALL-1 B-cell leukemia cells. Anti-BALL-1 effect of iPS-MP in vivo was demonstrated in a xeno-transplantation model using severe combined immunodeficient mice. In addition, we established a xeno-free culture protocol to generate iPS-DC and iPS-MP. Collectively, we demonstrated the possibility of application of iPS-DC and macrophages to cell therapy.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
References
Klimp AH, de Vries EG, Scherphof GL, Daemen T . A potential role of macrophage activation in the treatment of cancer. Crit Rev Oncol Hematol 2002; 44: 143–161.
Moore KJ, Fabunmi RP, Andersson LP, Freeman MW . In vitro-differentiated embryonic stem cell macrophages: a model system for studying atherosclerosis-associated macrophage functions. Arterioscler Thromb Vasc Biol 1998; 18: 1647–1654.
Fairchild PJ, Brook FA, Gardner RL, Graca L, Strong V, Tone Y et al. Directed differentiation of dendritic cells from mouse embryonic stem cells. Curr Biol 2000; 10: 1515–1518.
Lindmark H, Rosengren B, Hurt-Camejo E, Bruder CE . Gene expression profiling shows that macrophages derived from mouse embryonic stem cells is an improved in vitro model for studies of vascular disease. Exp Cell Res 2004; 300: 335–344.
Zhan X, Dravid G, Ye Z, Hammond H, Shamblott M, Gearhart J et al. Functional antigen-presenting leucocytes derived from human embryonic stem cells in vitro. Lancet 2004; 364: 163–171.
Slukvin II, Vodyanik MA, Thomson JA, Gumenyuk ME, Choi KD . Directed differentiation of human embryonic stem cells into functional dendritic cells through the myeloid pathway. J Immunol 2006; 176: 2924–2932.
Odegaard JI, Vats D, Zhang L, Ricardo-Gonzalez R, Smith KL, Sykes DB et al. Quantitative expansion of ES cell-derived myeloid progenitors capable of differentiating into macrophages. J Leukoc Biol 2007; 81: 711–719.
Su Z, Frye C, Bae KM, Kelley V, Vieweg J . Differentiation of human embryonic stem cells into immunostimulatory dendritic cells under feeder-free culture conditions. Clin Cancer Res 2008; 14: 6207–6217.
Tseng SY, Nishimoto KP, Silk KM, Majumdar AS, Dawes GN, Waldmann H et al. Generation of immunogenic dendritic cells from human embryonic stem cells without serum and feeder cells. Regen Med 2009; 4: 513–526.
Senju S, Hirata S, Matsuyoshi H, Masuda M, Uemura Y, Araki K et al. Generation and genetic modification of dendritic cells derived from mouse embryonic stem cells. Blood 2003; 101: 3501–3508.
Senju S, Suemori H, Zembutsu H, Uemura Y, Hirata S, Fukuma D et al. Genetically manipulated human embryonic stem cell-derived dendritic cells with immune regulatory function. Stem Cells 2007; 25: 2720–2729.
Matsuyoshi H, Senju S, Hirata S, Yoshitake Y, Uemura Y, Nishimura Y . Enhanced priming of antigen-specific CTLs in vivo by embryonic stem cell-derived dendritic cells expressing chemokine along with antigenic protein: application to antitumor vaccination. J Immunol 2004; 172: 776–786.
Matsuyoshi H, Hirata S, Yoshitake Y, Motomura Y, Fukuma D, Kurisaki A et al. Therapeutic effect of alpha-galactosylceramide-loaded dendritic cells genetically engineered to express SLC/CCL21 along with tumor antigen against peritoneally disseminated tumor cells. Cancer Sci 2005; 96: 889–896.
Fukuma D, Matsuyoshi H, Hirata S, Kurisaki A, Motomura Y, Yoshitake Y et al. Cancer prevention with semi-allogeneic ES cell-derived dendritic cells. Biochem Biophys Res Commun 2005; 335: 5–13.
Motomura Y, Senju S, Nakatsura T, Matsuyoshi H, Hirata S, Monji M et al. Embryonic stem cell-derived dendritic cells expressing glypican-3, a recently identified oncofetal antigen, induce protective immunity against highly metastatic mouse melanoma, B16-F10. Cancer Res 2006; 66: 2414–2422.
Matsunaga Y, Fukuma D, Hirata S, Fukushima S, Haruta M, Ikeda T et al. Activation of antigen-specific cytotoxic T lymphocytes by beta2-microglobulin or TAP1 gene disruption and the introduction of recipient-matched MHC class I gene in allogeneic embryonic stem cell-derived dendritic cells. J Immunol 2008; 181: 6635–6643.
Fukushima S, Hirata S, Motomura Y, Fukuma D, Matsunaga Y, Ikuta Y et al. Multiple antigen-targeted immunotherapy with alpha-galactosylceramide-loaded and genetically engineered dendritic cells derived from embryonic stem cells. J Immunother 2009; 32: 219–231.
Hirata S, Senju S, Matsuyoshi H, Fukuma D, Uemura Y, Nishimura Y . Prevention of experimental autoimmune encephalomyelitis by transfer of embryonic stem cell-derived dendritic cells expressing myelin oligodendrocyte glycoprotein peptide along with TRAIL or programmed death-1 ligand. J Immunol 2005; 174: 1888–1897.
Hirata S, Matsuyoshi H, Fukuma D, Kurisaki A, Uemura Y, Nishimura Y et al. Involvement of regulatory T cells in the experimental autoimmune encephalomyelitis-preventive effect of dendritic cells expressing myelin oligodendrocyte glycoprotein plus TRAIL. J Immunol 2007; 178: 918–925.
Takahashi K, Yamanaka S . Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell 2006; 126: 663–676.
Takahashi K, Tanabe K, Ohnuki M, Narita M, Ichisaka T, Tomoda K et al. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell 2007; 131: 861–872.
Yu J, Vodyanik MA, Smuga-Otto K, Antosiewicz-Bourget J, Frane JL, Tian S et al. Induced pluripotent stem cell lines derived from human somatic cells. Science 2007; 318: 1917–1920.
Park IH, Zhao R, West JA, Yabuuchi A, Huo H, Ince TA et al. Reprogramming of human somatic cells to pluripotency with defined factors. Nature 2008; 451: 141–146.
Lowry WE, Richter L, Yachechko R, Pyle AD, Tchieu J, Sridharan R et al. Generation of human induced pluripotent stem cells from dermal fibroblasts. Proc Natl Acad Sci USA 2008; 105: 2883–2888.
Senju S, Haruta M, Matsunaga Y, Fukushima S, Ikeda T, Takahashi K et al. Characterization of dendritic cells and macrophages generated by directed differentiation from mouse induced pluripotent stem cells. Stem Cells 2009; 27: 1021–1031.
Choi KD, Vodyanik MA, Slukvin, II . Generation of mature human myelomonocytic cells through expansion and differentiation of pluripotent stem cell-derived lin-CD34+CD43+CD45+ progenitors. J Clin Invest 2009; 119: 2818–2829.
Kopec KK, Carroll RT . Alzheimer’s beta-amyloid peptide 1–42 induces a phagocytic response in murine microglia. J Neurochem 1998; 71: 2123–2131.
Schuurman B, Heuff G, Beelen RH, Meyer S . Enhanced killing capacity of human Kupffer cells after activation with human granulocyte/macrophage-colony-stimulating factor and interferon gamma. Cancer Immunol Immunother 1994; 39: 179–184.
Lopez M, Bony V, Martinache C, Vincent F, Chokri M, Abina MA et al. Tumoricidal potential of human macrophages grown in vitro from blood monocytes. J Exp Ther Oncol 1996; 1: 143–154.
Baron-Bodo V, Doceur P, Lefebvre ML, Labroquere K, Defaye C, Cambouris C et al. Anti-tumor properties of human-activated macrophages produced in large scale for clinical application. Immunobiology 2005; 210: 267–277.
Glenner GG, Wong CW . Alzheimer’s disease and Down’s syndrome: sharing of a unique cerebrovascular amyloid fibril protein. Biochem Biophys Res Commun 1984; 122: 1131–1135.
Masters CL, Simms G, Weinman NA, Multhaup G, McDonald BL, Beyreuther K . Amyloid plaque core protein in Alzheimer disease and Down syndrome. Proc Natl Acad Sci USA 1985; 82: 4245–4249.
Levy E, Carman MD, Fernandez-Madrid IJ, Power MD, Lieberburg I, van Duinen SG et al. Mutation of the Alzheimer’s disease amyloid gene in hereditary cerebral hemorrhage, Dutch type. Science 1990; 248: 1124–1126.
Chen G, Chen KS, Knox J, Inglis J, Bernard A, Martin SJ et al. A learning deficit related to age and beta-amyloid plaques in a mouse model of Alzheimer’s disease. Nature 2000; 408: 975–979.
Tanzi RE, Bertram L . Twenty years of the Alzheimer’s disease amyloid hypothesis: a genetic perspective. Cell 2005; 120: 545–555.
Oddo S, Caccamo A, Shepherd JD, Murphy MP, Golde TE, Kayed R et al. Triple-transgenic model of Alzheimer’s disease with plaques and tangles: intracellular Abeta and synaptic dysfunction. Neuron 2003; 39: 409–421.
Leissring MA, Farris W, Chang AY, Walsh DM, Wu X, Sun X et al. Enhanced proteolysis of beta-amyloid in APP transgenic mice prevents plaque formation, secondary pathology, and premature death. Neuron 2003; 40: 1087–1093.
McLean CA, Cherny RA, Fraser FW, Fuller SJ, Smith MJ, Beyreuther K et al. Soluble pool of Abeta amyloid as a determinant of severity of neurodegeneration in Alzheimer’s disease. Ann Neurol 1999; 46: 860–866.
Lue LF, Kuo YM, Roher AE, Brachova L, Shen Y, Sue L et al. Soluble amyloid beta peptide concentration as a predictor of synaptic change in Alzheimer’s disease. Am J Pathol 1999; 155: 853–862.
Wang J, Dickson DW, Trojanowski JQ, Lee VM . The levels of soluble versus insoluble brain Abeta distinguish Alzheimer’s disease from normal and pathologic aging. Exp Neurol 1999; 158: 328–337.
Walsh DM, Selkoe DJ . A beta oligomers—a decade of discovery. J Neurochem 2007; 101: 1172–1184.
Simard AR, Soulet D, Gowing G, Julien JP, Rivest S . Bone marrow-derived microglia play a critical role in restricting senile plaque formation in Alzheimer’s disease. Neuron 2006; 49: 489–502.
El Khoury J, Toft M, Hickman SE, Means TK, Terada K, Geula C et al. Ccr2 deficiency impairs microglial accumulation and accelerates progression of Alzheimer-like disease. Nat Med 2007; 13: 432–438.
Barger SW, Harmon AD . Microglial activation by Alzheimer amyloid precursor protein and modulation by apolipoprotein E. Nature 1997; 388: 878–881.
Cagnin A, Brooks DJ, Kennedy AM, Gunn RN, Myers R, Turkheimer FE et al. In-vivo measurement of activated microglia in dementia. Lancet 2001; 358: 461–467.
Biglari A, Southgate TD, Fairbairn LJ, Gilham DE . Human monocytes expressing a CEA-specific chimeric CD64 receptor specifically target CEA-expressing tumour cells in vitro and in vivo. Gene Therapy 2006; 13: 602–610.
Nakatsuji N, Nakajima F, Tokunaga K . HLA-haplotype banking and iPS cells. Nat Biotechnol 2008; 26: 739–740.
Miyoshi H, Blomer U, Takahashi M, Gage FH, Verma IM . Development of a self-inactivating lentivirus vector. J Virol 1998; 72: 8150–8157.
Suemori H, Yasuchika K, Hasegawa K, Fujioka T, Tsuneyoshi N, Nakatsuji N . Efficient establishment of human embryonic stem cell lines and long-term maintenance with stable karyotype by enzymatic bulk passage. Biochem Biophys Res Commun 2006; 345: 926–932.
Tabata H, Kanai T, Yoshizumi H, Nishiyama S, Fujimoto S, Matsuda I et al. Characterization of self-glutamic acid decarboxylase 65-reactive CD4+ T-cell clones established from Japanese patients with insulin-dependent diabetes mellitus. Hum Immunol 1998; 59: 549–560.
Uemura Y, Senju S, Maenaka K, Iwai LK, Fujii S, Tabata H et al. Systematic analysis of the combinatorial nature of epitopes recognized by TCR leads to identification of mimicry epitopes for glutamic acid decarboxylase 65-specific TCRs. J Immunol 2003; 170: 947–960.
Niwa H, Masui S, Chambers I, Smith AG, Miyazaki J . Phenotypic complementation establishes requirements for specific POU domain and generic transactivation function of Oct-3/4 in embryonic stem cells. Mol Cell Biol 2002; 22: 1526–1536.
Watanabe K, Ueno M, Kamiya D, Nishiyama A, Matsumura M, Wataya T et al. A ROCK inhibitor permits survival of dissociated human embryonic stem cells. Nat Biotechnol 2007; 25: 681–686.
Acknowledgements
We thank Dr H Miyoshi for a lentivirus vector system and Dr H Niwa for a mammalian expression vector pCAGGS-IRES-PuroR. This work was supported in part by Grants-in-Aid 18014023, 19591172 and 19059012 from the Ministry of Education, Culture, Sports, Science, and Technology (MEXT), Japan, the Program of Founding Research Centers for Emerging Infectious Diseases launched as a project commissioned by MEXT, Research Grant for Intractable Diseases from Ministry of Health and Welfare, Japan, grants from Japan Science and Technology Agency (JST), the Uehara Memorial Foundation, and the Takeda Science Foundation.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Supplementary Information accompanies the paper on Gene Therapy website
Supplementary information
Rights and permissions
About this article
Cite this article
Senju, S., Haruta, M., Matsumura, K. et al. Generation of dendritic cells and macrophages from human induced pluripotent stem cells aiming at cell therapy. Gene Ther 18, 874–883 (2011). https://doi.org/10.1038/gt.2011.22
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/gt.2011.22
Keywords
This article is cited by
-
New cell sources for CAR-based immunotherapy
Biomarker Research (2023)
-
Induced pluripotent stem cell-derived dendritic cell vaccine therapy genetically modified on the ubiquitin-proteasome system
Gene Therapy (2023)
-
Induced pluripotent and CD34+ stem cell derived myeloid cells display differential responses to particle and dust mite exposure
Scientific Reports (2023)
-
Macrophages derived from pluripotent stem cells: prospective applications and research gaps
Cell & Bioscience (2022)
-
Tumor RNA transfected DCs derived from iPS cells elicit cytotoxicity against cancer cells induced from colorectal cancer patients in vitro
Scientific Reports (2022)