Abstract
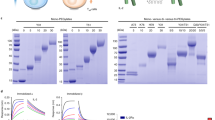
Therapeutic use and function of recombinant molecules can be studied by the expression of foreign genes in mice. In this study, we have expressed human Fcγ receptor–Ig fusion molecules (FcγR-Igs) in mice by administering FcγR-Ig plasmid DNAs hydrodynamically and compared their effectiveness with purified molecules in blocking immune-complex (IC)-mediated inflammation in mice. The concentration of hydrodynamically expressed FcγR-Igs (CD16AF-Ig, CD32AR-Ig and CD32AH-Ig) reached a maximum of 130 μg ml–1 of blood within 24 h after plasmid DNA administration. The in vivo half-life of FcγR-Igs was found to be 9–16 days and western blot analysis showed that the FcγR-Igs were expressed as a homodimer. The hydrodynamically expressed FcγR-Igs blocked 50–80% of IC-mediated inflammation up to 3 days in a reverse passive Arthus reaction model. Comparative analysis with purified molecules showed that hydrodynamically expressed FcγR-Igs are more efficient than purified molecules in blocking IC-mediated inflammation and had a higher half-life. In summary, these results suggest that the administration of a plasmid vector with the FcγR-Ig gene can be used to study the consequences of blocking IC binding to FcγRs during the development of inflammatory diseases. This approach may have potential therapeutic value in treating IC-mediated inflammatory autoimmune diseases such as lupus, arthritis and autoimmune vasculitis.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
References
Liu F, Song YK, Liu D . Hydrodynamics-based transfection in animals by systemic administration of plasmid DNA. Gene Therapy 1999; 6: 1258–1266.
Zhang G, Budker V, Wolff JA . High levels of foreign gene expression in hepatocytes after tail vein injections of naked plasmid DNA. Hum Gene Ther 1999; 10: 1735–1737.
Suda T, Liu D . Hydrodynamic gene delivery. Its principles and applications. Mol Ther 2007; 15: 2063–2069.
Herweijer H, Wolff JA . Gene therapy progress and prospects. Hydrodynamic gene delivery. Gene Therapy 2007; 14: 99–107.
Budker VG, Subbotin VM, Budker T, Sebestyen MG, Zhang G, Wolff JA . Mechanism of plasmid delivery by hydrodynamic tail vein injection. II. Morphological studies. J Gene Med 2006; 8: 874–888.
Andrianaivo F, Lecocq M, Wattiaux-De Conick S, Wattiaux R, Jadot M . Hydrodynamics-based transfection of the liver: entrance into hepatocytes of DNA that causes expression takes place very early after injection. J Gene Med 2004; 6: 877–883.
Kobayashi N, Kuramoto T, Yamaoka K, Hashida M, Takakura Y . Hepatic uptake and gene expression mechanisms following intravenous administration of plasmid DNA by conventional and hydrodynamics-based procedures. J Pharmacol Exp Ther 2001; 297: 853–860.
Chang HE, Hanawa H, Liu H, Yoshida T, Hayashi M, Watanabe R et al. Hydrodynamic-based delivery of an interleukin-22-Ig fusion gene ameliorates experimental autoimmune myocarditis in rats. J Immunol 2006; 177: 3635–3643.
Chang HE, Hanawa H, Yoshida T, Hayashi M, Liu H, Ding L et al. Alteration of IL-17 related expressions in experimental autoimmune myocarditis and inhibition of Il-17 by IL-10-Ig fusion gene transfer. Circ J 2008; 72: 813–819.
Abe S, Hanawa H, Hayashi M, Yoshida T, Komura S, Watanabe R et al. Prevention of experimental autoimmune myocarditis by hydrodynamics-based naked plasmid DNA encoding CTLA4-Ig gene delivery. J Card Fail 2005; 11: 557–564.
Matsui Y, Inobe M, Okamoto H, Chiba S, Shimizu T, Kitabatake A . Blockade of T cell costimulatory signals using adenovirus vectors prevents both the induction and the progression of experimental autoimmune myocarditis. J Mol Cell Cardiol 2002; 34: 279–295.
Kawaguchi Y . A gene therapy or purified CTLA4IgG treatment of experimental allergic encephalomyelitis. Hokkaido Igaku Zasshi 1999; 74: 467–475.
Mihara M, Tan I, Chuzhin Y, Reddy B, Budhai L, Holzer A et al. CTLA4Ig inhibits T cell-dependent B-cell maturation in murine systemic lupus erythematosus. J Clin Invest 2000; 106: 91–101.
Quattrocchi E, Dallman MJ, Feldmann M . Adenovirus-mediated gene transfer of CTLA-4Ig fusion protein in the suppression of experimental autoimmune arthritis. Arthritis Rheum 2000; 43: 1688–1697.
Wu X, He Y, Falo Jr L, Hui K, Huang L . Regression of human mammary adenocarcinoma by systemic administration of a recombinant gene encoding the hFlex-TRAIL fusion protein. Mol Ther 2001; 3: 368–374.
Yazawa H, Murakami T, Li H-M, Back T, Kurosaka K, Suzuki Y et al. Hydrodynamics-based gene delivery of naked DNA encoding fetal liver kinase-1 gene effectively suppresses the growth of pre-existing tumors. Cancer Gene Ther 2006; 13: 993–1001.
Neal ZC, Bates MK, Albertini MR, Herweijer H . Hydrodynamics limb vein delivery of a xenogeneic DNA cancer vaccine effectively induces antitumor immunity. Mol Ther 2007; 15: 422–430.
Hulett MD, Hogarth PM . Molecular basis of Fc receptor function. Adv Immunol 1994; 57: 1–127.
Kimberly RP, Salmon JE, Edberg JC . Receptors for immunoglobulin G. Molecular diversity and implications for disease. Arthritis Rheum 1995; 38: 306–314.
Ravetch JV . Fc receptors. Curr Opin Immunol 1997; 9: 121–125.
Unkeless JC, Scigliano E, Freedman VH . Structure and function of human and murine receptors for IgG. Annu Rev Immunol 1988; 6: 251–281.
Selvaraj P, Fifadara N, Nagarajan S, Cimino A, Wang G . Functional regulation of human neutrophil Fc gamma receptors. Immunol Res 2004; 29: 219–230.
Anderson CL, Looney RJ . Human leukocyte IgG Fc receptors. Immunol Today 1986; 7: 264–266.
Fanger MW, Shen L, Graziano RF, Guyre PM . Cytotoxicity mediated by human Fc receptors for IgG. Immunol Today 1989; 10: 92–99.
Hogarth PM . Fc receptors are major mediators of antibody-based inflammation in autoimmunity. Curr Opin Immunol 2002; 14: 798–802.
Takai T . Fc receptors and their role in immune regulation and autoimmunity. J Clin Immunol 2005; 25: 1–18.
Schmidt RE, Gessner JE . Fc receptors and their interaction with complement in autoimmunity. Immunol Lett 2005; 100: 56–67.
Takai T, Li M, Sylvestre D, Clynes R, Ravetch JV . FcR gamma chain deletion results in pleiotropic effector cell defects. Cell 1994; 76: 519–529.
Ravetch JV, Clynes RA . Divergent roles for Fc receptors and complement in vivo. Annu Rev Immunol 1998; 16: 421–432.
Clynes R, Dumitru C, Ravetch JV . Uncoupling of immune complex formation and kidney damage in autoimmune glomerulonephritis. Science 1998; 279: 1052–1054.
Hazenbos WL, Gessner JE, Hofhuis FM, Kuipers H, Meyer D, Heijnen IA et al. Impaired IgG-dependent anaphylaxis and Arthus reaction in Fc gamma RIII (CD16) deficient mice. Immunity 1996; 5: 181–188.
Clarkson SB, Bussel JB, Kimberly RP, Valinsky JE, Nachman RL, Unkeless JC . Treatment of refractory immune thrombocytopenic purpura with anti-Fcγ receptor antibody. New Engl J Med 1986; 314: 1236–1239.
Ravetch JV, Bolland S . IgG Fc receptors. Annu Rev Immunol 2001; 19: 275–290.
Takai T, Nakamura A, Akiyama K . Fc receptors as potential targets for the treatment of allergy, autoimmune disease and cancer. Curr Drug Targets Immune Endocr Metabol Disord 2003; 3: 187–197.
Cohen-Solal JF, Cassard L, Fridman WH, Sautes-Fridman C . Fc gamma receptors. Immunol Lett 2004; 92: 199–205.
Tarzi RM, Cook HT . Role of Fc gamma receptors in glomerulonephritis. Nephron Exp Nephrol 2003; 95: e7–12.
Nakamura A, Takai T . A role of Fc gamma RIIB in the development of collagen-induced arthritis. Biomed Pharmacother 2004; 58: 292–298.
Shashidharamurthy R, Hennigar RA, Fuchs S, Palaniswami P, Sherman M, Selvaraj P . Extravasations and emigration of neutrophils to the inflammatory site depend on the interaction of immune-complex with Fc gamma receptors and can be effectively blocked by decoy Fc gamma receptors. Blood 2008; 111: 894–904.
Arthus M . Injections repetees de serum de cheval chez le lapin. C R Soc Biol 1903; 55: 817.
Li P, Nagarajan S, Zhu C, Selvaraj P . Recombinant CD16A-Ig forms a homodimer and cross-blocks the ligand binding functions of neutrophil and monocyte Fcγ receptors. Mol Immunol 2002; 38: 527–538.
Shashidharamurthy R, Zhang F, Amano A, Kamat A, Panchanathan R, Ezekwudo D et al. Dynamics of the interaction of human IgG subtypes immune complexes with cells expressing R and H allelic forms of a low affinity Fcγ receptor CD32A. J Immunol 2009; 183: 8216–8224.
Ignatovich AI, Dizhes EB, Palvotskayan AV, Akifiev BN, Burov SV, Orlov SV et al. Complexes of plasmid DNA with basic domain 47-57 of the HIV-1 tat protein are transferred to mammalian cells by endocytosis-mediated pathways. J Biol Chem 2003; 278: 42625–42636.
van Sorge NM, van der Pol W-L, Van de Winkel JGJ . FcγR polymorphisms: implications for function, disease susceptibility and immunotherapy. Tissue Antigens 2003; 61: 189–202.
Dijstelbloem HM, Bijl M, Fijnheer R, Scheepers RH, Oost WW, Jansen MD et al. Fcγ receptor polymorphisms in systemic lupus erythematosus: association with disease and in vivo clearance of immune complexes. Arthritis Rheum 2000; 43: 2793–2800.
Ludo van der Pol W, van de winkel GJ . IgG receptor polymorphism: risk factor for disease. Immunogen 1998; 48: 222–232.
Tan SY . FcγRIIa polymorphism in systemic lupus erythematosus. Kidney Blood Press Res 2000; 23: 138–142.
Manger K, Repp R, Spriewald BM, Rascu A, Geiger A, Wassmuth R et al. Fcγ receptor IIa polymorphism in Caucasian patients with systemic lupus erythromatosus: association with clinical symptoms. Arthritis Rheum 1998; 41: 1181–1189.
Ierino FL, Powell MS, McKenzie IFC, Hogarth PM . Recombinant soluble human Fc-gamma-RII: production, characterization, and inhibition of the Arthus reaction. J Exp Med 1993; 178: 1617–1628.
Ellsworth JL, Hamacher N, Harder B, Bannink K, Bukowski TR, Byrnes-Blake K et al. Recombinant soluble human FcγR1A (CD64A) reduces inflammation in murine collagen induced arthritis. J Immunol 2009; 182: 7272–7279.
Junghans RP, Anderson CL . The protection receptor for IgG catabolism is the β2-microglobulin-containing neonatal intestinal transport receptor. Proc Natl Acad Sci USA 1996; 93: 5512–5516.
Chappel MS, Isenman DE, Everett M, Xu YY, Dorrington KJ, Klein MH . Identification of the Fcγ receptor class I binding site in human IgG through the use of recombinant IgG1/IgG2 hybrid and point-mutated antibodies. Proc Natl Acad Sci USA 1991; 88: 9036–9040.
Chappel MS, Isenman DE, Oomen R, Xu YY, Klein MH . Identification of a secondary FcγRI binding site within a genetically engineered human IgG antibody. J Biol Chem 1993; 268: 25124–25131.
Ledbetter JA, Gilliand LK, Hayden MS, Linsley PS, Bajorath J, Fell HP . Expression vectors encoding bispecific fusion proteins and methods of producing biologically active bispecific fusion proteins in mammalian cells. United States Patent 2000: 6132992.
Tao MH, Morrison SL . Studies of aglycosylated chimeric mouse-human IgG. Role of carbohydrate in the structure and effector functions mediated by the human IgG constant region. J Immunol 1989; 143: 2595–2601.
Roopenian DC, Akilesh S . FcRn: the neonatal Fc receptor comes of age. Nat Rev Immunol 2007; 7: 715–725.
He Y, Pimenov AA, Nayak JV, Plowey J, Falo LD, Huang L . Intravenous injection of naked DNA encoding secreted flt3 ligand dramatically increases the number of dendritic cells and natural killer cells in vivo. Hum Gene Ther 2000; 11: 547.
Acknowledgements
This study was supported by the NIH Grants R21HL09126802 and R21AR05682101 to PS and the AHA grant 11SDG5710004 to RS. We also thank Ms Archana Boopathy, Ms Sumi Selvaraj and Ms Danielle Daniels for critical reading of the article.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Rights and permissions
About this article
Cite this article
Shashidharamurthy, R., Machiah, D., Bozeman, E. et al. Hydrodynamic delivery of plasmid DNA encoding human FcγR-Ig dimers blocks immune-complex mediated inflammation in mice. Gene Ther 19, 877–885 (2012). https://doi.org/10.1038/gt.2011.175
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/gt.2011.175
Keywords
This article is cited by
-
Synergic silencing of costimulatory molecules prevents cardiac allograft rejection
Journal of Translational Medicine (2014)