Abstract
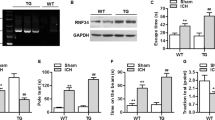
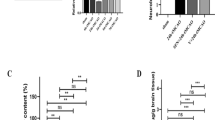
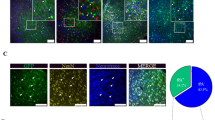
To study the effects of excision repair cross-complementing 1 (ERCC1) on the pathophysiological process of brain ischemia, we examined the changes in ERCC1 expression, as well as the functional significance of ERCC1 in the rat brain following middle cerebral artery occlusion (MCAO). The results were as follows: (1) ERCC1 immunopositive cells were widely distributed in various brain regions. ERCC1 expression was localized to the nuclei of neurons and astrocytes. (2) ERCC1 expression, as determined by western blot, increased at 3 days, remaining until 14 days, in the ipsilateral cortex and striatum following MCAO. Immunohistochemical analysis demonstrated that ischemia induced increased ERCC1 expression within the periinfarct core, with increasingly less expression toward the core. (3) Knockdown of ERCC1 expression by intraventricular injection of antisense plasmids increased DNA damage and infarct volume in the ischemic brain. (4) ERCC1 overproduction, by injection of expression plasmids, significantly reduced infarct volume and the accumulation of DNA-damaged neurons. Taken together, these results indicate that both endogenous ERCC1 and exogenous ERCC1 have an important neuroprotective function in the brain. In addition, administration of ERCC1 to the brain could prove to be a successful strategy for neuronal protection against ischemic injury.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
Abbreviations
- BER:
-
base excision repair
- DSB:
-
DNA double-strand break
- ERCC1:
-
excision repair cross-complementing 1
- IP:
-
ischemic preconditioning
- MCAO:
-
middle cerebral artery occlusion
- NER:
-
nucleotide excision repair
- PCNA:
-
proliferating cell nuclear antigen
- SSB:
-
DNA single-strand break
References
Liu PK, Hsu CY, Dizdaroglu M, Floyd RA, Kow YW, Karakaya A et al. Damage, repair, and mutagenesis in nuclear genes after mouse forebrain ischemia-reperfusion. J Neurosci 1996; 16: 6795–6806.
Nagayama T, Lan J, Henshall DC, Chen D, O’Horo C, Simon RP et al. Induction of oxidative DNA damage in the peri-infarct region after permanent focal cerebral ischemia. J Neurochem 2000; 75: 1716–1728.
Nguyen T, Brunson D, Crespi CL, Penman BW, Wishnok JS, Tannenbaum SR . DNA damage and mutation in human cells exposed to nitric oxide in vitro. Proc Natl Acad Sci USA 1992; 89: 3030–3034.
Payne CM, Bernstein C, Bernstein H . Apoptosis overview emphasizing the role of oxidative stress, DNA damage and signal-transduction pathways. Leuk Lymphoma 1995; 19: 43–93.
Chen J, Jin K, Chen M, Pei W, Kawaguchi K, Greenberg DA et al. Early detection of DNA strand breaks in the brain after transient focal ischemia: implications for the role of DNA damage in apoptosis and neuronal cell death. J Neurochem 1997; 69: 232–245.
Kawase M, Fujimura M, Morita-Fujimura Y, Chan PH . Reduction of apurinic/apyrimidinic endonuclease expression after transient global cerebral ischemia in rats: implication of the failure of DNA repair in neuronal apoptosis. Stroke 1999; 30: 441–448; discussion 449.
Tomasevic G, Kamme F, Wieloch T . Changes in proliferating cell nuclear antigen, a protein involved in DNA repair, in vulnerable hippocampal neurons following global cerebral ischemia. Brain Res Mol Brain Res 1998; 60: 168–176.
Imai H, Harland J, McCulloch J, Graham DI, Brown SM, Macrae IM . Specific expression of the cell cycle regulation proteins, GADD34 and PCNA, in the peri-infarct zone after focal cerebral ischaemia in the rat. Eur J Neurosci 2002; 15: 1929–1936.
Li N, Wu H, Yang S, Chen D . Ischemic preconditioning induces XRCC1, DNA polymerase-beta, and DNA ligase III and correlates with enhanced base excision repair. DNA Repair (Amst) 2007.
Chen D, Minami M, Henshall DC, Meller R, Kisby G, Simon RP . Upregulation of mitochondrial base-excision repair capability within rat brain after brief ischemia. J Cereb Blood Flow Metab 2003; 23: 88–98.
Lan J, Li W, Zhang F, Sun FY, Nagayama T, O’Horo C et al. Inducible repair of oxidative DNA lesions in the rat brain after transient focal ischemia and reperfusion. J Cereb Blood Flow Metab 2003; 23: 1324–1339.
Sugawara T, Noshita N, Lewen A, Kim GW, Chan PH . Neuronal expression of the DNA repair protein Ku 70 after ischemic preconditioning corresponds to tolerance to global cerebral ischemia. Stroke 2001; 32: 2388–2393.
Sun FY, Lin X, Mao LZ, Ge WH, Zhang LM, Huang YL et al. Neuroprotection by melatonin against ischemic neuronal injury associated with modulation of DNA damage and repair in the rat following a transient cerebral ischemia. J Pineal Res 2002; 33: 48–56.
Yang ZJ, Bao WL, Qiu MH, Zhang LM, Lu SD, Huang YL et al. Role of vascular endothelial growth factor in neuronal DNA damage and repair in rat brain following a transient cerebral ischemia. J Neurosci Res 2002; 70: 140–149.
Ling X, Zhang LM, Huang YL, Bao WL, Sun FY . Neuronal ERCC6 mRNA expression in rat brain induced by a transient focal cerebral ischemia. Zhongguo Yao Li Xue Bao 1999; 20: 15–20.
Li W, Luo Y, Zhang F, Signore AP, Gobbel GT, Simon RP et al. Ischemic preconditioning in the rat brain enhances the repair of endogenous oxidative DNA damage by activating the base-excision repair pathway. J Cereb Blood Flow Metab 2006; 26: 181–198.
De Laat WL, Jaspers NG, Hoeijmakers JH . Molecular mechanism of nucleotide excision repair. Genes Dev 1999; 13: 768–785.
Murai M, Enokido Y, Inamura N, Yoshino M, Nakatsu Y, van der Horst GT et al. Early postnatal ataxia and abnormal cerebellar development in mice lacking Xeroderma pigmentosum Group A and Cockayne syndrome Group B DNA repair genes. Proc Natl Acad Sci USA 2001; 98: 13379–13384.
Wood RD, Mitchell M, Sgouros J, Lindahl T . Human DNA repair genes. Science 2001; 291: 1284–1289.
Matsunaga T, Mu D, Park CH, Reardon JT, Sancar A . Human DNA repair excision nuclease. Analysis of the roles of the subunits involved in dual incisions by using anti-XPG and anti-ERCC1 antibodies. J Biol Chem 1995; 270: 20862–20869.
Ferry KV, Hamilton TC, Johnson SW . Increased nucleotide excision repair in cisplatin-resistant ovarian cancer cells: role of ERCC1-XPF. Biochem Pharmacol 2000; 60: 1305–1313.
Selvakumaran M, Pisarcik DA, Bao R, Yeung AT, Hamilton TC . Enhanced cisplatin cytotoxicity by disturbing the nucleotide excision repair pathway in ovarian cancer cell lines. Cancer Res 2003; 63: 1311–1316.
Motycka TA, Bessho T, Post SM, Sung P, Tomkinson AE . Physical and functional interaction between the XPF/ERCC1 endonuclease and hRad52. J Biol Chem 2004; 279: 13634–13639.
Niedernhofer LJ, Odijk H, Budzowska M, van Drunen E, Maas A, Theil AF et al. The structure-specific endonuclease Ercc1-Xpf is required to resolve DNA interstrand cross-link-induced double-strand breaks. Mol Cell Biol 2004; 24: 5776–5787.
Kuraoka I, Kobertz WR, Ariza RR, Biggerstaff M, Essigmann JM, Wood RD . Repair of an interstrand DNA cross-link initiated by ERCC1-XPF repair/recombination nuclease. J Biol Chem 2000; 275: 26632–26636.
Sargent RG, Meservy JL, Perkins BD, Kilburn AE, Intody Z, Adair GM et al. Role of the nucleotide excision repair gene ERCC1 in formation of recombination-dependent rearrangements in mammalian cells. Nucleic Acids Res 2000; 28: 3771–3778.
Adair GM, Rolig RL, Moore-Faver D, Zabelshansky M, Wilson JH, Nairn RS . Role of ERCC1 in removal of long non-homologous tails during targeted homologous recombination. EMBO J 2000; 19: 5552–5561.
Schrader CE, Vardo J, Linehan E, Twarog MZ, Niedernhofer LJ, Hoeijmakers JH et al. Deletion of the nucleotide excision repair gene Ercc1 reduces immunoglobulin class switching and alters mutations near switch recombination junctions. J Exp Med 2004; 200: 321–330.
Selfridge J, Hsia KT, Redhead NJ, Melton DW . Correction of liver dysfunction in DNA repair-deficient mice with an ERCC1 transgene. Nucleic Acids Res 2001; 29: 4541–4550.
Zou LL, Huang L, Hayes RL, Black C, Qiu YH, Perez-Polo JR et al. Liposome-mediated NGF gene transfection following neuronal injury: potential therapeutic applications. Gene Therapy 1999; 6: 994–1005.
Zhang R, Xue YY, Lu SD, Wang Y, Zhang LM, Huang YL et al. Bcl-2 enhances neurogenesis and inhibits apoptosis of newborn neurons in adult rat brain following a transient middle cerebral artery occlusion. Neurobiol Dis 2006; 24: 345–356.
Wang YQ, Guo X, Qiu MH, Feng XY, Sun FY . VEGF overexpression enhances striatal neurogenesis in brain of adult rat after a transient middle cerebral artery occlusion. J Neurosci Res 2007; 85: 73–82.
Longa EZ, Weinstein PR, Carlson S, Cummins R . Reversible middle cerebral artery occlusion without craniectomy in rats. Stroke 1989; 20: 84–91.
Paxinos G, Watson C . The Rat Brain in Stereotaxic Coordinates, 2nd edn. Academic Press: Orlando, 1986.
Schmued LC, Hopkins KJ . Fluoro-Jade B: a high affinity fluorescent marker for the localization of neuronal degeneration. Brain Res 2000; 874: 123–130.
Swanson RA, Morton MT, Tsao-Wu G, Savalos RA, Davidson C, Sharp FR . A semiautomated method for measuring brain infarct volume. J Cereb Blood Flow Metab 1990; 10: 290–293.
Acknowledgements
This work was supported in part by grants from the National Basic Research Program of China (nos. 2006CB504100 and 2006CB943702), National Natural Science Foundation of China (no. 30770660) and Shanghai Metropolitan Fund for Research and Development (nos. 04DZ14005 and 07DZ14005).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
He, KY., Yang, SZ., Shen, DH. et al. Excision repair cross-complementing 1 expression protects against ischemic injury following middle cerebral artery occlusion in the rat brain. Gene Ther 16, 840–848 (2009). https://doi.org/10.1038/gt.2009.48
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/gt.2009.48