Abstract
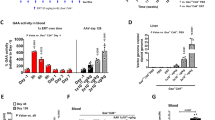
Mutant proteins have the potential to exert dominant-negative effects that might limit the therapeutic efficacy of their wild-type counterparts after gene transfer. For ornithine transcarbamylase (OTC) deficiency, in vitro studies have suggested the presence of dominant-negative effects, however, supporting in vivo studies have not been conducted. In this study, we exploited the capacity of recombinant adeno-associated virus (rAAV) 2/8 vectors to deliver transgenes to the mouse liver with high efficiency to determine whether expression of selected OTC mutant proteins exert inhibitory effects on endogenous wild-type OTC enzymatic activity. Using site-directed mutagenesis we constructed three OTC mutants with a theoretical or reported in vitro capacity to exert dominant-negative effects, and delivered these to the liver using rAAV2/8. Each mutation had been earlier identified in patients with OTC deficiency. Treated mice showed no increase in urinary orotic acid levels or reduction in OTC activity despite supra-physiological expression of the mutant proteins, consistent with an absence of dominant-negative effects. These data have important implications for the development of gene therapy strategies for OTC deficiency and validate a model system in which potential dominant-negative effects of specific mutations in prospective patients can be examined empirically before gene therapy.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
Accession codes
References
Brusilow SW, Horwich AL . Urea cycle enzymes. In: Scriver CR, Beaudet AL, Sly WS, Valle D (eds.) The Metabolic and Molecular Bases of Inherited Disease. McGraw-Hill Inc.: New York, 1995, pp 118–1232.
Tuchman M, Jaleel N, Morizono H, Sheehy L, Lynch MG . Mutations and polymorphisms in the human ornithine transcarbamylase gene. Hum Mutat 2002; 19: 93–107.
Morsy MA, Zhao JZ, Ngo TT, Warman AW, O'Brien WE, Graham FL et al. Patient selection may affect gene therapy success. Dominant negative effects observed for ornithine transcarbamylase in mouse and human hepatocytes. J Clin Invest 1996; 97: 826–832.
Horwich AL, Kalousek F, Fenton WA, Pollock RA, Rosenberg LE . Targeting of pre-ornithine transcarbamylase to mitochondria: definition of critical regions and residues in the leader peptide. Cell 1986; 44: 451–459.
Alexander IE, Cunningham SC, Logan GJ, Christodoulou J . Potential of AAV vectors in the treatment of metabolic disease. Gene Therapy 2008; 15: 831–839.
Cunningham SC, Spinoulas A, Carpenter KH, Wilcken B, Kuchel PW, Alexander IE . AAV2/8-mediated correction of OTC deficiency is robust in adult but not neonatal spfash mice. Mol Ther (in press).
Grompe M, Caskey CT, Fenwick RG . Improved molecular diagnostics for ornithine transcarbamylase deficiency. Am J Hum Genet 1991; 48: 212–222.
Maddalena A, Spence JE, O'Brien WE, Nussbaum RL . Characterization of point mutations in the same arginine codon in three unrelated patients with ornithine transcarbamylase deficiency. J Clin Invest 1988; 82: 1353–1358.
Reish O, Plante RJ, Tuchman M . Four new mutations in the ornithine transcarbamylase gene. Biochem Med Metab Biol 1993; 50: 169–175.
Shi D, Morizono H, Ha Y, Aoyagi M, Tuchman M, Allewell NM . 1.85-A resolution crystal structure of human ornithine transcarbamoylase complexed with N-phosphonacetyl-L-ornithine. Catalytic mechanism and correlation with inherited deficiency. J Biol Chem 1998; 273: 34247–34254.
Valentini G, De Gregorio A, Di Salvo C, Grimm R, Bellocco E, Cuzzocrea G et al. An essential lysine in the substrate-binding site of ornithine carbamoyltransferase. Eur J Biochem 1996; 239: 397–402.
Cunningham SC, Dane AP, Spinoulas A, Logan GJ, Alexander IE . Gene delivery to the juvenile mouse liver using AAV2/8 vectors. Mol Ther 2008; 16: 1081–1088.
Wallace R, Knecht E, Grisolia S . Turnover of rat liver ornithine transcarbamylase. FEBS Lett 1986; 208: 427–430.
Xiao X, Li J, Samulski RJ . Production of high-titer recombinant adeno-associated virus vectors in the absence of helper adenovirus. J Virol 1998; 72: 2224–2232.
Gorman C . High efficiency gene transfer into mammalian cells. In: Glover DM (ed.). DNA cloning. IRL Press: Oxford, Washington DC, 198, pp 143–165.
Ye X, Robinson MB, Batshaw ML, Furth EE, Smith I, Wilson JM . Prolonged metabolic correction in adult ornithine transcarbamylase-deficient mice with adenoviral vectors. J Biol Chem 1996; 271: 3639–3646.
Acknowledgements
We thank Professor James M Wilson (Department of Pathology and Laboratory Medicine, University of Pennsylvania, USA) for kindly providing the AAV8 serotype plasmid p5E18-VD2/8, Professor R Jude Samulski (Gene Therapy Center, The University of North Carolina at Chapel Hill, USA) for providing the packaging plasmid pXX6 and Professor Matthew During (Department of Medical and Health Science, University of Auckland, New Zealand) for providing the pAM2 construct. We also thank Professor Nick Hoogenraad (Department of Biochemistry and Molecular Sciences, La Trobe University, Victoria, Australia) for providing the human OTC antibody, Allison Dane for help with injections and Grant Logan for critical reading of the manuscript. This work was supported by a project grant from the NHMRC (423400). Samantha L Ginn is the recipient of a fellowship honouring the memory of Noel Dowling.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ginn, S., Cunningham, S., Zheng, M. et al. In vivo assessment of mutations in OTC for dominant-negative effects following rAAV2/8-mediated gene delivery to the mouse liver. Gene Ther 16, 820–823 (2009). https://doi.org/10.1038/gt.2009.38
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/gt.2009.38