Abstract
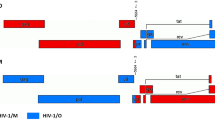
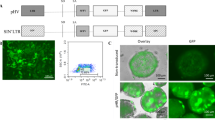
Recombinant Semliki Forest virus (SFV) is an attractive viral vector system owing to its ability to allow high efficiency of viral protein expression. To produce recombinant pseudotyped human immunodeficiency virus type 1 (HIV-1) virions, we designed a chimeric SFV/HIV vector system that contains both the HIV-1 cis- and trans-acting elements under the transcriptional control of the SFV replicase and investigated the ability of the hybrid SFV/HIV system to produce lentiviral particles capable of transducing target cells. Co-transfection of target cells with the two helper SFV packaging system RNAs along with each SFV/Gag-Pol, SFV/VSVG as well as SFV/HIV-1 vector unit replicon led to the generation of efficient transducing competent recombinant SFV/HIV particles. In contrast, co-transduction of target cells with the SFV/HIV chimeric virions produced recombinant particles with low transducing ability. Our data suggest that both the genomic and the subgenomic RNAs containing the HIV-1 vector unit were negatively selected for incorporation into recombinant particles, despite the fact that the SFV-driven HIV-1 vector replicon was the only one containing a lentiviral packaging sequence. The results of this study provide insights relevant to the design of chimeric lentiviral vectors.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Naldini L, Blömer U, Gallay P, Ory D, Mulligan R, Gage FH et al. In vivo gene delivery and stable transduction of nondividing cells by a lentiviral vector. Science 1996; 272: 263–267.
Vigna E, Naldini L . Lentiviral vectors: excellent tools for experimental gene transfer and promising candidates for gene therapy. J Gene Med 2000; 2: 308–316.
Sirven A, Pflumio F, Zennou V, Titeux M, Vainchenker W, Coulombel L et al. The human immunodeficiency virus type-1 central DNA flap is a crucial determinant for lentiviral vector nuclear import and gene transduction of human hematopoietic stem cells. Blood 2000; 96: 4103–4110.
Blömer U, Naldini L, Kafri T, Trono D, Verma IM, Gage FH . Highly efficient and sustained gene transfer in adult neurons with a lentivirus vector. J Virol 1997; 71: 6641–6649.
Kafri T, Blömer U, Peterson DA, Gage FH, Verma IM . Sustained expression of genes delivered directly into liver and muscle by lentiviral vectors. Nat Biotechnol 1997; 17: 314–317.
Delenda C . Lentiviral vectors: optimization of packaging, transduction and gene expression. J Gene Med 2004; 6: S125–S138.
Morris KV . VRX-496(VIRxSYS). Curr Opin Investig Drugs 2005; 6: 209–215.
Kafri T, van Praag H, Ouyang L, Gage FH, Verma IM . A packaging cell line for lentivirus vectors. J Virol 1999; 73: 576–584.
Strang BL, Ikeda Y, Cosset FL, Collins MK, Takeuchi Y . Characterization of HIV-1 vectors with gammaretrovirus envelope glycoproteins produced from stable packaging cells. Gene Therapy 2004; 11: 591–598.
Strang BL, Takeuchi Y, Relander T, Richter J, Bailey R, Sanders DA et al. Human immunodeficiency virus type 1 vectors with alphavirus envelope glycoproteins produced from stable packaging cells. J Virol 2005; 79: 1765–1771.
Broussau S, Jabbour N, Lachapelle G, Durocher Y, Tom R, Transfiguracion J et al. Inducible packaging cells for large-scale production of lentiviral vectors in serum-free suspension culture. Mol Ther 2008; 16: 500–507.
Miyoshi H, Blömer U, Takahashi M, Gage FH, Verma IM . Development of a self-inactivating lentivirus vector. J Virol 1998; 72: 8150–8157.
Gasmi M, Glynn J, Jin MJ, Jolly DJ, Yee JK, Chen ST . Requirements for efficient production and transduction of human immunodeficiency virus type 1-based vectors. J Virol 1999; 73: 1828–1834.
Dull T, Zufferey R, Kelly M, Mandel RJ, Nguyen M, Trono D et al. A third-generation lentivirus vector with a conditional packaging system. J Virol 1998; 72: 8463–8471.
Kim VN, Mitrophanous K, Kingsman SM, Kingsman AJ . Minimal requirement for a lentivirus vector based on human immunodeficiency virus type 1. J Virol 1998; 72: 811–816.
Zufferey R, Nagy D, Mandel RJ, Naldini L, Trono D . Multiply attenuated lentiviral vector achieves efficient gene delivery in vivo. Nat Biotechnol 1997; 15: 871–875.
Kotsopoulou E, Kim VN, Kingsman AJ, Kingsman SM, Mitrophanous KA . A Rev-independent human immunodeficiency virus type 1 (HIV-1)-based vector that exploits a codon-optimized HIV-1 gag-pol gene. J Virol 2000; 74: 4839–4852.
Pollard VW, Malim MH . The HIV-1 Rev protein. Annu Rev Microbiol 1998; 52: 491–532.
Parolin C, Dorfman T, Palú G, Göttlinger H, Sodroski J . Analysis in human immunodeficiency virus type 1 vectors of cis-acting sequences that affect gene transfer into human lymphocytes. J Virol 1994; 68: 3888–3895.
Srinivasakumar N, Schuening FG . A lentivirus packaging system based on alternative RNA transport mechanisms to express helper and gene transfer vector RNAs and its use to study the requirement of accessory proteins for particle formation and gene delivery. J Virol 1999; 11: 9589–9598.
Mautino MR, Keiser N, Morgan RA . Improved titers of HIV-based lentiviral vectors using the SRV-1 constitutive transport element. Gene Therapy 2000; 16: 1421–1424.
Konetschny C, Holzer GW, Urban C, Hämmerle T, Mayrhofer J, Falkner FG . Generation of transduction-competent retroviral vectors by infection with a single hybrid vaccinia virus. J Virol 2003; 12: 7017–7025.
Kong W, Tian C, Liu B, Yu XF . Stable expression of primary human immunodeficiency virus type 1 structural gene products by use of a noncytopathic sindbis virus vector. J Virol 2002; 76: 11434–11439.
Strauss JH, Strauss EG . The alphaviruses: gene expression, replication, and evolution. Microbiol Rev 1994; 58: 491–562.
Merten OW . State-of-the-art of the production of retroviral vectors. J Gene Med 2004; 6: S105–S124.
Lundstrom K . Semliki Forest virus vectors for rapid and high-level expression of integral membrane proteins. Biochim Biophys Acta 2003; 1610: 90–96.
Pushko P, Parker M, Ludwig GV, Davis NL, Johnston RE, Smith JF . Replicon-helper systems from attenuated Venezuelan equine encephalitis virus: expression of heterologous genes in vitro and immunization against heterologous pathogens in vivo. Virology 1997; 239: 389–401.
Smerdou C, Liljestrom P . Two-helper RNA system for production of recombinant Semliki Forest virus particles. J Virol 1999; 73: 1092–1098.
Liljestrom P, Garoff H . A new generation of animal cell expression vectors based on the Semliki Forest virus replicon. Biotechnology 1991; 9: 1356–1361.
Li KJ, Garoff H . Production of infectious recombinant Moloney murine leukemia virus particles in BHK cells using Semliki Forest virus-derived RNA expression vectors. Proc Natl Acad Sci USA 1996; 93: 11658–11663.
Wahlfors JJ, Xanthopoulos KG, Morgan RA . Semliki Forest virus-mediated production of retroviral vector RNA in retroviral packaging cells. Hum Gene Ther 1997; 17: 2031–2041.
Wahlfors JJ, Morgan RA . Production of minigene-containing retroviral vectors using an alphavirus/retrovirus hybrid vector system. Hum Gene Ther 1999; 10: 1197–1206.
Lebedeva I, Fujita K, Nihrane A, Silver J . Infectious particles derived from Semliki Forest virus vectors encoding murine leukemia virus envelopes. J Virol 1997; 71: 7061–7067.
Piver E, Collin C, Renault N, Bru T, Pagès JC . Mobilization of full-length Semliki Forest virus replicon by retrovirus particles. J Virol 2006; 19: 9889–9895.
Burns JC, Friedmann T, Driever W, Burrascano M, Yee JK . Vesicular stomatitis virus G glycoprotein pseudotyped retroviral vectors: concentration to very high titer and efficient gene transfer into mammalian and nonmammalian cells. Proc Natl Acad Sci USA 1993; 90: 8033–8037.
Perri S, Driver D, Gardner JP, Sherrill S, Belli B, Dubensky Jr TW et al. Replicon vectors derived from Sindbis virus and Semliki Forest virus that establish persistent replication in host cells. J Virol 2000; 74: 9802–9807.
Delenda C, Gaillard C . Real-time quantitative PCR for the design of lentiviral vector analytical assays. Gene Therapy 2005; 12: S36–S50.
Rohr UP, Wulf MA, Stahn S, Steidl U, Haas R, Kronenwett R . Fast and reliable titration of recombinant adeno-associated virus type-2 using quantitative real-time PCR. J Virol Methods 2002; 106: 81–88.
Sastry L, Johnson T, Hobson M, Smucker B, Cornetta K . Titering lentiviral vectors: comparison of DNA, RNA and marker expression methods. Gene Therapy 2002; 9: 1155–1162.
Holzer GW, Mayrhofer JA, Gritschenberger W, Dorner F, Falkner FG . Poxviral/retroviral chimeric vectors allow cytoplasmic production of transducing defective retroviral particles. Virology 1999; 253: 107–114.
Muriaux D, Mirro J, Harvin D, Rein A . RNA is a structural element in retrovirus particles. Proc Natl Acad Sci USA 2001; 9: 5246–5251.
Perri S, Greer CE, Thudium K, Doe B, Legg H, Liu H et al. An alphavirus replicon particle chimera derived from Venezuelan equine encephalitis and sindbis viruses is a potent gene-based vaccine delivery vector. J Virol 2003; 77: 10394–10403.
Paessler S, Fayzulin RZ, Anishchenko M, Greene IP, Weaver SC, Frolov I . Recombinant sindbis/Venezuelan equine encephalitis virus is highly attenuated and immunogenic. J Virol 2003; 77: 9278–9286.
Rulli Jr SJ, Hibbert CS, Mirro J, Pederson T, Biswal S, Rein A . Selective and nonselective packaging of cellular RNAs in retrovirus particles. J Virol 2007; 81: 6623–6631.
Houzet L, Paillart JC, Smagulova F, Maurel S, Morichaud Z, Marquet R et al. HIV controls the selective packaging of genomic, spliced viral and cellular RNAs into virions through different mechanisms. Nucleic Acid Res 2007; 35: 2695–2704.
Berglund JA, Charpentier B, Rosbash M . A high affinity binding site for the HIV-1 nucleocapsid protein. Nucleic Acid Res 1997; 25: 1042–1049.
Clever JL, Taplitz RA, Lochrie MA, Polisky B, Parslow TG . A heterologous, high-affinity RNA ligand for human immunodeficiency virus Gag protein has RNA packaging activity. J Virol 2000; 74: 541–546.
Laham-Karam N, Bacharach E . Transduction of human immunodeficiency virus type 1 vectors lacking encapsidation and dimerization signals. J Virol 2007; 81: 10687–10698.
Dorange F, Piver E, Bru T, Collin C, Roingeard P, Pages JC . Vesicular stomatitis virus glycoprotein: a transducing coat for SFV-based RNA vectors. J Gene Med 2004; 6: 1014–1022.
Rolls MM, Webster P, Balba NH, Rose JK . Novel infectious particles generated by expression of the vesicular stomatitis virus glycoprotein from a self-replicating RNA. Cell 1994; 79: 497–506.
Rolls MM, Haglund K, Rose JK . Expression of additional genes in a vector derived from a minimal RNA virus. Virology 1996; 218: 406–411.
Clever JL, Miranda Jr D, Parslow TG . RNA structure and packaging signals in the 5′ leader region of the human immunodeficiency virus type 1 genome. J Virol 2002; 76: 12381–12387.
Kujala P, Ikäheimonen A, Ehsani N, Vihinen H, Auvinen P, Kääriäinen L . Biogenesis of the Semliki Forest virus RNA replication complex. J Virol 2001; 75: 3873–3884.
Brandt S, Blißenbach M, Grewe B, Konietzny R, Grunwald T, Überla K . Rev proteins of human and simian immunodeficiency virus enhance RNA encapsidation. Plos Pathog 2007; 3: e54.
Poole E, Strappe P, Mok HP, Hicks R, Lever AM . HIV-1 Gag–RNA interaction occurs at a perinuclear/centrosomal site; analysis by confocal microscopy and FRET. Traffic 2005; 6: 741–755.
Sfakianos JN, Hunter E . PMV capsid transport is mediated by Env/Gag interactions at the pericentriolar recycling endosome. Traffic 2003; 4: 671–680.
Swanson CM, Puffer BA, Ahmad KM, Doms RW, Malim MH . Retroviral mRNA nuclear export elements regulate protein function and virion assembly. EMBO J 2004; 23: 2632–2640.
Piver E, Collin C, Diatta A, Vaudin P, Pagès JC . Cellular factors influencing Semliki Forest Virus vector biology. Gene Therapy 2005; 12: S111–S117.
Lundstrom K . Biology and application of alphaviruses in gene therapy. Gene Therapy 2005; 12: S92–S97.
Wahlfors JJ, Zullo SA, Loimas S, Nelson DM, Morgan RA . Evaluation of recombinant alphaviruses as vectors in gene therapy. Gene Therapy 2000; 7: 472–480.
Krieg PA, Melton DA . In vitro RNA synthesis with SP6 RNA polymerase. Methods Enzymol 1987; 155: 397–415.
Martin-Rendon E, White LJ, Olsen A, Mitrophanous KA, Mazarakis ND . New methods to titrate EIAV-based lentiviral vectors. Mol Ther 2002; 5: 566–570.
Accola MA, Strack B, Gottlinger HG . Efficient particle production by minimal gag constructs which retain the carboxy-terminal domain of human immunodeficiency virus type 1 capsid-p2 and a late assembly domain. J Virol 2000; 74: 5395–5402.
Parolin C, Taddeo B, Palú G, Sodroski J . Use of cis- and trans-acting viral regulatory sequences to improve expression of human immunodeficiency virus vectors in human lymphocytes. Virology 1996; 222: 415–422.
Acknowledgements
This work was supported by grants from Regione Veneto, MIUR (PRIN-2005), Istituto Superiore di Sanità (Rome-AIDS Project nos. 30G.24 and 30G.55) and University of Padova (Ex 60% and ATENEO 2006) to AC, GP and CP. MC is a PhD student in the Virology and Microbial Biotechnologies program at the University of Padova. We thank F Salandin for artwork.
Author information
Authors and Affiliations
Corresponding author
Additional information
This paper is dedicated to the memory of R Parolin.
Rights and permissions
About this article
Cite this article
Del Vecchio, C., Calistri, A., Lombardi, G. et al. Analysis of human immunodeficiency virus type 1 vector cis- and trans-acting elements production by means of Semliki Forest virus. Gene Ther 16, 279–290 (2009). https://doi.org/10.1038/gt.2008.159
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/gt.2008.159