Abstract
Purpose
Farber disease (OMIM 22800) is an ultrarare progressive multisystemic neurodevelopmental storage disorder caused by a deficiency of the lysosomal enzyme acid ceramidase (AC). Hard clinical end points for future clinical trials remain to be defined.
Methods
We quantitatively analyzed published cases with Farber disease (N = 96). The main outcome variables were survival and diagnostic delay. As a potential predictor of survival, the influence of residual AC enzyme activity was investigated. The analysis was performed in compliance with STROBE criteria.
Results
The median survival period of the study population was 3 years. The median age at disease onset was 3 months, and the median age at diagnosis was 17 months. The median diagnostic delay was 13.75 months. Patients with residual AC activity in fibroblasts at more than 5.1% of the normal level survived significantly longer than patients with residual AC activity below this threshold. In addition, higher residual AC activity was associated with a later onset of symptoms.
Conclusion
Farber disease onset is in infancy. Diagnostic delay is typically substantial. Our data suggest a phenotype-biomarker association with implications for future clinical and therapeutic trials. In the absence of a prospective multicenter natural-history study protocol, we believe that our modeling approach, based on published case descriptions, is the best and most timely approximation for generalizability.
Similar content being viewed by others
Introduction
Farber disease (OMIM 22800) is an autosomal recessive ultrarare progressive neurodevelopmental storage disorder caused by mutations in the gene ASAH1 localized on chromosome 8p22, leading to a deficiency of the lysosomal enzyme acid N-acylsphingosine amidohydrolyase 1 (AC), which is also termed ceramidase.1, 2 The impaired breakdown of sphingolipids leads to ceramide accumulation in various cell types (e.g., histiocytes, epithelial cells, Schwann cells) of organs such as the skin, kidney, liver, lungs, and brain, thereby inducing a multisystemic and often lethal phenotype.3
Farber disease was first reported as a disease entity in 1952, by Sidney Farber, who described three patients with disseminated lipogranulomatosis.4 The prevalence and incidence of Farber disease (ORPHA 333) are not precisely known; the epidemiological Orphanet report of November 2016 lists 80 known cases.5 Novel therapies are in development. Recombinant human acid ceramidase, an enzyme replacement therapy, was given orphan designation by the European Medicines Agency on 14 February 2014, and is in the preclinical phase of development (http://roivant.com/#pipeline, accessed 6 March 2017).6 Orphan drug designation was not granted by the US Food and Drug Administration until March 2017.7 One observational biomarker study is listed on ClinicalTrials.gov (NCT02298634, accessed 1 March 2017).
As with many orphan diseases, even those for which treatment is available, little is known about the natural history of Farber disease. A thorough understanding of its natural history will be helpful in assessing the impact of specific therapies and can reduce uncertainty in regard to counseling afflicted families. Given the rarity of the condition, conducting a long-term, worldwide prospective natural-history study would be difficult. Valuable information, especially on hard clinical end points, can be obtained from systematic analysis of published cases in the literature.8, 9 We therefore directed our efforts toward analyzing the onset of disease, survival rates, clinical characteristics, and biomarker-phenotype descriptions of the evidence on Farber disease published as clinical case descriptions or case series. In addition, we analyzed the geographical distribution of patients worldwide, which may be of interest for epidemiological reasons and for the planning of future clinical trials.
Materials and methods
This analysis was conducted in compliance with STROBE (Strengthening the Reporting of Observational Studies in Epidemiology) criteria.10
Literature review and definitions of variables
We performed a comprehensive literature search on PubMed with the keywords “Farber disease,” “Farber lipogranulomatosis,” and “acid ceramidase deficiency.” Identified publications were downloaded (N = 196) and sorted for reports containing relevant clinical, biochemical, and genetic information (N = 99). The reports were published between 1952 and 2016. Overall, we identified 96 patients from 97 publications with sufficient information for analysis (Supplementary Figure 1 online). Papers were published in English, German, or French. The database closure date was 30 December 2016. None of the analyzed patients participated in enzymatic replacement therapy trials, nor did they undergo bone-marrow transplantation.
The following variables were extracted: age at onset; age at diagnosis; symptoms leading to diagnosis; last reported age; information on whether the patient is alive or deceased; AC activity in leukocytes, fibroblasts, liver, or kidney; intracellular ceramide storage in fibroblasts, liver, or kidney; publication year; and geographic origin of patients. If the origin of a patient was not explicitly stated, the country was assumed to be that of the first author’s institutional affiliation in the case description.
If information regarding time was given in semiquantitative terms, we took a conservative approach and defined the findings as follows: “stillborn” = day 0, “newborn” or “at birth” = day 1, “newborn period” = 1 month, “postmortem” = age at death.
Statistical analysis
Techniques of descriptive statistics were applied as reported previously.8, 9 Variables were illustrated using counts and percentages of the total study population. Since biochemical parameters (enzymatic activity, levels of intracellular AC content) were measured in different laboratories, values were expressed as percentages of the mean of the reported normal range. Survival was defined as the time difference between patient birth and age at death. If the patient was reported to be alive at the time of the last follow-up visit, patient data were censored. Survival was estimated using the Kaplan-Meier method. The log-rank test was applied to compare potential differences between the analyzed subgroups. We used unbiased recursive partitioning to determine cutoff values for the impact of residual AC activity and year of publication on survival findings in subgroup analyses.11 Diagnostic delay was calculated as the difference between age at diagnosis and age at onset of disease. To determine whether there exist distinct disease subtypes in Farber disease or whether, instead, there is a continuous spectrum of phenotype, we performed a cluster analysis of reported signs and symptoms, using the Ward algorithm. The world map was plotted using the R extension ggmap.12
Missing data were not imputed. Sensitivity analyses were not conducted. All analyses were conducted using R (http://www.r-project.org). P values reported were two-sided. P ≤ 0.05 was considered statistically significant.
Results
We identified 96 patients from 81 families (using data published between 1952 and 2016) for further statistical analysis. Within the study cohort, 91 patients were diagnosed postnatally owing to the presentation of characteristic features, while in 3 patients the diagnosis of Farber disease was established prenatally. For 2 patients the time of diagnosis was not stated. The characteristics of the study sample are depicted in Table 1. The origins of affected individuals are shown in Figure 1. Because data were missing in specific subsets, patients had to be excluded for different subsequent data analyses; sample sizes are always indicated (n) for the corresponding analyses.
Prenatally diagnosed cohort
In the prenatally diagnosed group, two out of three patients were diagnosed prenatally by family screening due to a prior affected sibling. Both pregnancies were terminated after medical consultation. The third patient manifested with progressive intrauterine growth retardation and loss of fetal movements and was diagnosed after intrauterine fetal death postmortem by characteristic ultrastructural findings (i.e., foamy macrophages in light microscopy and Farber bodies visualized by electronic microscopy) in autopsy.
Postnatally diagnosed cohort
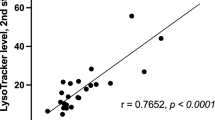
Descriptive statistical values are illustrated in Supplementary Table 1. In the group of postnatally diagnosed patients, median age at onset of disease was 3 months, interquartile range from 1 to 9 months (n = 86). Median age at diagnosis was 17 months, interquartile range from 9.25 to 36 months (n = 86). Median diagnostic delay was 13.75 months, interquartile range from 6 to 29.5 months (n = 86) (Figure 2). The median estimated survival of the cohort of postnatally diagnosed patients (n = 91) was 3 years (Figure 3a). Interestingly, patients with a residual AC enzyme activity above 5.1% in fibroblasts survived significantly longer compared to patients whose residual enzymatic AC activity was 5.1% of normal or below (P = 0.0007, log-rank test; n = 24; threshold determined by unbiased recursive partitioning) (Figure 3b). In addition, a higher residual AC activity in fibroblasts was associated with later onset of disease (P = 0.0225, linear regression; n = 24) (Figure 3c). The level of intracellular ceramide storage had no impact on survival, in either analyzed tissue (Kaplan-Meier; n = 11) or age at onset of disease (linear regression; n = 6).
Estimated survival distributions and age at disease onset for patients with Farber disease. (a): Estimated overall survival distribution for patients with Farber disease (n = 91). (b) Estimated survival distribution for Farber disease patients with a residual AC enzyme activity above 5.1% (n = 10, thick line) and below or equal to 5.1% of normal controls (n = 14, thin line). Enzymatic activity was measured in fibroblasts. “+” is used to mark censored individuals. Log-rank test, P = 0.0007. (c) Age at disease onset (in years) of Farber disease patients subject to residual AC activity (% of normal). Enzymatic activity was measured in fibroblasts. Each point represents a single patient (n = 24), gray dashed line displays estimated regression curve. Linear regression, slope = 0.29, P = 0.0225.
We analyzed the distribution of presenting or leading signs or symptoms in the study sample (Supplementary Table 2). The most common features of Farber disease were subcutaneous nodules, arthritis or joint swelling, hoarseness, flexion contractures, failure to thrive, and developmental delay or cognitive impairment. Clinical feature clustering is shown in Figure 4. Of note, survival rates did not differ between the cohort of patients exhibiting neurological symptoms and those lacking signs of neurological affection. In addition, the number of symptoms reported in an affected individual had no influence on the survival time. We analyzed survival rates of patients published before and after the year 2000 (threshold determined by unbiased recursive partitioning). Patients whose data were published after 2000 had a significantly longer survival rate than that in the cohort whose data were published before 2000. However, when taking into account the reported residual AC activity, the proportion of patients having a residual AC activity above 5.1% was higher in the cohort with data published after 2000 (n = 13) than in the group of patients with data published before 2000 (n = 1).
Clinical feature clustering of patients with Farber disease. (a) Dendrogram: clinical symptom clustering. Each number indicates assignment to a specific group. Analysis was performed using a Ward fusion algorithm. (b) Relative frequency of symptoms per group. Groups 1 and 2 exhibit similar generalized disease features, with more frequent phenotypical manifestations in group 1 than in group 2. Group 3 appeared more exclusive, with epilepsy and amyotrophy. There was no clear-cut exclusive delineation of phenotypical features across patients.
Discussion
In a worldwide cohort of 96 patients with Farber disease, we quantitatively delineated age at disease onset, estimated survival, seminal clinical features, diagnostic delay, and a biomarker-phenotype correlation; i.e., patients with residual AC enzyme activity above 5.1% in fibroblasts survived significantly longer than did patients whose residual enzymatic AC activity was 5.1% of normal or below.
A classification into subtypes was proposed for Farber disease.3 Cluster analysis allowed the mathematical distinction of three statistically similar subgroups. Clinically, groups 1 and 2 appeared to exhibit similar generalized disease features with more frequent phenotypical manifestations in group 1 than in group 2, whereas in group 3 the disease appeared to be more distinct, with epilepsy and amyotrophy as its cardinal features. However, as there was no clear-cut exclusive delineation of phenotypical features across patients, there seems to be a continuous phenotypic spectrum, rather than distinct well-defined subtypes, in Farber disease. This is consistent with observations in other lysosomal disorders, such as Gaucher disease.13 Owing to the rarity of the disease, diagnostic suspicion may be difficult, as illustrated by the median diagnostic delay in the present cohort of 13.75 months after disease manifestation. What should alert the clinician is the triad of subcutaneous nodules, joint swellings/arthritis, and hoarse or weak voice.3 These classic features were reported in 88% (subcutaneous nodules), 86% (joint swellings/arthritis), and 74% (hoarse/weak voice) of patients in the present study population. The clinical phenotype is broad, which is consistent with a multisystemic, progressive storage disease, and includes the involvement of various organ systems such as (i) the brain (developmental delay/cognitive impairment, neurodegeneration, amyotrophy, muscular hypotonia, brain atrophy, epilepsy), (ii) the eye (cherry-red spot, nystagmus, cloudy cornea), (iii) the internal organs (organomegaly), (iv) physical appearance (dysmorphic features), and (v) the musculoskeletal system (flexion contractures, osteoporosis and other bone involvement). Given the variety of combinations of symptoms reported in the present study cohort, Farber disease should be suspected not only in individuals presenting with the classic clinical triad but also in any patient exhibiting an overlapping pattern of neurological and rheumatological manifestations (outlined in Supplementary Table 2) that, per se, appear unspecific in isolation but become suggestive of an underlying neurogenetic disease when appearing in combination in a given individual. Of note, in contrast to other lysosomal storage disorders,9 hydrops fetalis as the very first clinical feature is rare (3%) in Farber disease.
As in other rare diseases,8, 9 the diagnostic delay in Farber disease is still substantial, as shown by our results. Early diagnosis is important, since in many diseases early diagnosis leads to a better outcome, especially when the diagnosis is established in the “therapeutic window of opportunity,” which results in the reversibility of morbidity or prevention of morbidity and premature mortality.
There are single published cases of hematopoietic stem-cell transplantations in patients with Farber disease, documenting partial initial therapeutic response but ongoing neurological progression in most cases.14, 15, 16, 17 Preclinical gene therapy studies were carried out in mouse models and enzymatically normal nonhuman primates.18, 19 Furthermore, enzyme replacement therapy with Chinese hamster ovary cell–derived recombinant human AC was tested in a mouse model.20 As of 15 September 2017, ClinicalTrials.gov did not list any open therapeutic trials for humans. In general, enzyme replacement therapies in lysosomal storage disorders work well on organomegaly. The effect on connective tissue and bone may be limited, and peripherally injected enzymes do not cross the blood–brain barrier, owing to the size of the molecule. In addition, early initiation of therapy before irreversible damage has occurred is an important principle.21 Time to diagnosis is the surrogate sign for disease awareness and may decrease over time, especially if clinical trials commence or if a specific therapy is available in the future, because the condition will be considered treatable and might be added to diagnostic algorithm tools such as Treatable-ID.22 Besides raising disease awareness, screening specific populations at risk within patient groups with commonly encountered conditions that may phenotypically overlap with Farber disease—e.g., rheumatologic disorders, developmental delay, organomegaly, and bone disease—may be another method of reducing time to diagnosis in this rare condition.23
Patients whose data were published after 2000 lived longer compared with patients with data published earlier, but this phenomenon was probably biased by the fact that a substantially higher proportion of data for less severely affected patients (i.e., those with residual AC activity above 5.1%) was published after 2000. An effect of improved standard of care is therefore less likely, but not impossible. The geographical distribution pattern of reported patients suggests a panethnic pattern across all inhabited continents and includes developed and developing countries.
Limitations and directions for future research
As previously described in similar studies, the present methodology has several very important limitations.8, 9 Because case reports often focus on a specific aspect of the disease, standardized quantitative descriptions of softer variables (e.g., joint mobility, skin nodules, quality of life) are subject to ascertainment bias and missing data. Laboratory data were pooled as samples and were not analyzed in a central laboratory and according to a standardized protocol. Biomarker–phenotype correlations described in the present work should therefore be considered somewhat exploratory and, ideally, confirmed in prospective clinical studies. Of note, biomarkers reflect the activity of a disease process24 and can be of diagnostic, prognostic, predictive, or pharmacodynamic nature.9, 25 Specifically, AC activity in fibroblasts would be considered a prognostic biomarker, based on the present findings. In contrast, biomarkers serving as truly validated surrogate markers are required to capture the severity of disease and reflect the net effect of the treatment on the true outcome,26 which can be challenging to demonstrate in a rare disease.27
Survival data may be in part historic and may change over time. Improved supportive medical care would prolong life. A more palliative, medically less aggressive approach centered on quality of life may decrease survival time.
Despite the limitations listed above, our approach is of value, especially as multicenter prospective natural-history studies would require a substantial international effort as well as many years to complete in order to obtain similar information on hard clinical end points. The geographic distribution of patients may be of help in identifying study centers for future clinical research. Of course, the study of softer end points (e.g., seizure types and frequency, range of joint motion, hepatic and splenic volumes, nodule size, and, in particular, biomarkers) is best conducted in prospective trials. There may be various levels of softer clinical end points within a broad spectrum of clinical impact, affecting—in ascending order of significance—quality of life (skin nodules), physical functioning (joint range of motion), neurological functioning (seizures), and developmental and neurocognitive outcomes.
Conclusion
We defined important clinical characteristics, including hard clinical end points, in a global population of 96 patients with Farber disease. In the absence of a prospective, multicenter natural-history study protocol, we believe that the modeling approach described in the present paper and others8, 9 is the best and most timely approximation to generalizability.
References
Sugita M, Dulaney JT, Moser HW . Ceramidase deficiency in Farber’s disease (lipogranulomatosis). Science 1972;178:1100–1102.
Koch J, Gartner S, Li CM et al. Molecular cloning and characterization of a full-length complementary DNA encoding human acid ceramidase. Identification of the first molecular lesion causing Farber disease. J Biol Chem 1996;271:33110–33115.
Levade T, Sandhoff K, Schulze H, Medin JA . 143: acid ceramidase deficiency: Farber lipogranulomatosis In: Valle D (ed). The Online Metabolic and Molecular Bases of Inherited Disease 2017. http://ommbid.mhmedical.com/content.aspx?bookid=971§ionid=62643272. Accessed 10 April 2017.
Farber S . A lipid metabolic disorder: disseminated lipogranulomatosis; a syndrome with similarity to, and important difference from, Niemann-Pick and Hand-Schuller-Christian disease. AMA Am J Dis Child 1952;84:499–500.
Orphanet Report Series. Prevalence of rare diseases: Bibliographic data. November 2016, no. 1, June 2016; http://www.orpha.net/orphacom/cahiers/docs/GB/Prevalence_of_rare_diseases_by_alphabetical_list.pdf. Accessed 1 March 2017.
European Medicines Agency. Public summary of opinion on orphan designation. Recombinant human acid ceramidase for the treatment of Farber disease, 2014. http://www.ema.europa.eu/docs/en_GB/document_library/Orphan_designation/2014/03/WC500164316.pdf. Accessed 1 March 2017.
US Food and Drug Administration. Search Orphan Drug Designations and Approvals, 2017. https://www.accessdata.fda.gov/scripts/opdlisting/oopd/. Accessed 1 March 2017.
Mechler K, Mountford WK, Hoffmann GF, Ries M . Ultra-orphan diseases: a quantitative analysis of the natural history of molybdenum cofactor deficiency. Genet Med 2015;17:965–970.
Zielonka M, Garbade SF, Kolker S, Hoffmann GF, Ries M . Quantitative clinical characteristics of 53 patients with MPS VII: a cross-sectional analysis. Genet Med 2017;19:983–988.
von Elm E, Altman DG, Egger M et al. The strengthening the reporting of observational studies in epidemiology (STROBE) statement: guidelines for reporting observational studies. Lancet 2007;370:1453–1457.
Hothorn T, Hornik K, Zeileis A . Unbiased recursive partitioning: a conditional inference framework. J Comput Graph Stat 2006;15:651–674.
Kahle D, Wickham H . ggmap: spatial visualization with ggplot2. R J 2013;5:144–161.
Sidransky E . Gaucher disease: complexity in a “simple” disorder. MolGenet Metab 2004;83:6–15.
Cappellari AM, Torcoletti M, Triulzi F, Corona F . Nervous system involvement in Farber disease. J Inherit Metab Dis 2016;39:149–150.
Yeager AM, Uhas KA, Coles CD, Davis PC, Krause WL, Moser HW . Bone marrow transplantation for infantile ceramidase deficiency (Farber disease). Bone Marrow Transplant 2000;26:357–363.
Jarisch A, Steward CG, Sorensen J et al. Odontoid infiltration and spinal compression in Farber disease: reversal by haematopoietic stem cell transplantation. Eur J Pediatr 2014;173:1399–1403.
Torcoletti M, Petaccia A, Pinto RM et al. Farber disease in infancy resembling juvenile idiopathic arthritis: identification of two new mutations and a good early response to allogeneic haematopoietic stem cell transplantation. Rheumatology (Oxford) 2014;53:1533–1534.
Ramsubir S, Nonaka T, Girbes CB, Carpentier S, Levade T, Medin JA . In vivo delivery of human acid ceramidase via cord blood transplantation and direct injection of lentivirus as novel treatment approaches for Farber disease. Mol Genet Metab 2008;95:133–141.
Walia JS, Neschadim A, Lopez-Perez O et al. Autologous transplantation of lentivector/acid ceramidase-transduced hematopoietic cells in nonhuman primates. Hum Gene Ther 2011;22:679–687.
He X, Dworski S, Zhu C et al. Enzyme replacement therapy for Farber disease: Proof-of-concept studies in cells and mice. BBA Clin 2017;7:85–96.
Ries M . Enzyme replacement therapy and beyond—in memoriam Roscoe O. Brady, M.D. (1923–2016). J Inherit Metab Dis 2017;40:343–356.
van Karnebeek CD, Houben RF, Lafek M, Giannasi W, Stockler S . The treatable intellectual disability APP www.treatable-id.org: a digital tool to enhance diagnosis & care for rare diseases. Orphanet J Rare Dis 2012;7:47.
Kastner B, Behre S, Lutz N et al. Clinical Research in Vulnerable Populations: Variability and Focus of Institutional Review Boards’ Responses. PLoS One 2015;10:e0135997.
Katz R . Biomarkers and surrogate markers: an FDA perspective. NeuroRx 2004;1:189–195.
US Food and Drug Administration. Center for Drug Evaluation and Research. Guidance for Industry and FDA Staff: Qualification Process for Drug Development Tools, 2014. http://www.fda.gov/downloads/Drugs/GuidanceComplianceRegulatoryInformation/Guidances/UCM230597.pdf. Accessed 19 June 2017.
Prentice RL . Surrogate endpoints in clinical trials: definition and operational criteria. Stat Med 1989;8:431–440.
Schiffmann R, Ries M, Blankenship D et al. Changes in plasma and urine globotriaosylceramide levels do not predict Fabry disease progression over 1 year of agalsidase alfa. Genet Med 2013;15:983–989.
Acknowledgements
M.Z. received support from the Physician-Scientist Program at Ruprecht-Karls-University Heidelberg Faculty of Medicine.
Author contributions
Matthias Zielonka: study design, data acquisition and interpretation, drafting and revision of the manuscript. Sven F. Garbade: statistical analysis, drafting and revision of the manuscript. Stefan Kölker: revision of the manuscript. Georg F. Hoffmann: revision of the manuscript. Markus Ries: study design, data interpretation, drafting and revision of the manuscript. All authors contributed to the critical revision of the manuscript for intellectual content and gave final approval for the version to be published.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
M.R. received consultancy fees or research grants from Alexion, GSK, Oxyrane, and Shire. The other authors declare no conflicts of interest.
Additional information
Supplementary material is linked to the online version of the paper at
Rights and permissions
About this article
Cite this article
Zielonka, M., Garbade, S., Kölker, S. et al. A cross-sectional quantitative analysis of the natural history of Farber disease: an ultra-orphan condition with rheumatologic and neurological cardinal disease features. Genet Med 20, 524–530 (2018). https://doi.org/10.1038/gim.2017.133
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/gim.2017.133
Keywords
This article is cited by
-
Spinal Muscular Atrophy with Progressive Myoclonic Epilepsy (SMA-PME): three new cases and review of the mutational spectrum
Italian Journal of Pediatrics (2023)
-
rAAV-mediated over-expression of acid ceramidase prevents retinopathy in a mouse model of Farber lipogranulomatosis
Gene Therapy (2023)
-
Diagnostic delay in rare diseases: data from the Spanish rare diseases patient registry
Orphanet Journal of Rare Diseases (2022)
-
Disease awareness or subtle product placement? Orphan diseases featured in the television series “House, M.D.” - a cross-sectional analysis
BMC Medical Ethics (2020)
-
Hepatic pathology and altered gene transcription in a murine model of acid ceramidase deficiency
Laboratory Investigation (2019)