Abstract
Purpose
To compare the efficacy and safety of conbercept and ranibizumab when administered according to a treat-and-extend (TREX) protocol for the treatment of neovascular age-related macular degeneration (AMD) in China.
Patients and methods
Between May 2014 and May 2015, 180 patients were treated in a 1 : 1 ratio using conbercept or ranibizumab from four hospitals. Patients received either conbercept 0.5 mg or ranibizumab 0.5 mg intravitreal injections. Follow-up time was 1 year and treated based on a TREX approach. Main outcomes and measures include best-corrected visual acuity (BCVA), using Early Treatment Diabetic Retinopathy Study (ETDRS); number of injections; central retinal thickness (CRT); and leakage of choroidal neovascularization before and after the treatment was analyzed by fluorescein fundus angiography and indocyanine green angiography.
Results
The 1-year visit was completed by 168 (93.3%) of patients. Mean BCVA was equivalent between two cohorts, and were improved by 12.7±7.770 and 12.3±7.269 letters in the conbercept and ranibizumab cohorts, respectively (P=0.624). There was no significant difference in measured CRT, with a mean decrease of 191.5 μm for conbercept and 187.8 μm for ranibizumab (P=0.773). There was a statistically significant difference (P=0.001) between the drugs regarding the number of treatments: 7.4 for conbercept and 8.7 for ranibizumab. The difference in the distribution of injection intervals was statistically significant between two groups (P=0.011). During the study, there were no cases of endophthalmitis or intraocular inflammation.
Conclusion
Both drugs had equivalent effects in visual and anatomic gains at 1 year when administered. In the conbercept group, longer treatment intervals were achieved with more patients.
Similar content being viewed by others
Introduction
Age-related macular degeneration (AMD) is one of the leading causes of legal blindness in the elderly population of Western countries.1 In recent years, the incidence of AMD in China also showed an upward trend, with the prevalence in some developed areas being close to the level of Western developed countries.2, 3 Choroidal neovascularization (CNV), based on which the neovascular AMD is defined, has been used to measure the severity of neovascular AMD.4 Vascular endothelial growth factor (VEGF) promotes the development and growth of CNV membranes.5, 6 Exudation and hemorrhage cause a thickening of the central retina, which when untreated can progress to scar formation and loss of vision.5 Initial therapies to treat neovascular AMD include fundus laser, transpupillary thermotherapy and photodynamic therapy. Since the advent of anti-VEGF medications in 2005, the treatment of neovascular AMD entered an era with superior results.6
Because of the important role that VEGF plays in the pathogenesis of AMD, VEGF has become the main target of CNV treatment at present.7 Before 2011, most widely used pharmaceutical agents were ranibizumab (Lucentis; Genentech, Inc., South San Francisco, CA, USA), which was approved by the Food and Drug Administration, and bevacizumab (Avastin; Genentech Inc.). Both of these drugs are monoclonal antibodies that block VEGF-A.8, 9, 10 In 2011, aflibercept (Eylea; Regeneron, Inc., Tarrytown, NJ, USA) was approved as a VEGF receptor fusion protein to treat neovascular AMD in the USA. Aflibercept works as a multi-target VEGF family blocker and binds isoforms of VEGF-A, VEGF-B, and placenta growth factor (PlGF).11 Conbercept (Langmu; Kanghong, Inc., Sichuan, China) is a different VEGF receptor (VEGFR) fusion protein. It blocks all isoforms of VEGF-A, VEGF-B, VEGF-C, and PlGF. It has a high binding affinity to VEGF and a long half-life in vitreous.12 In late 2013, it received the new drug certificate, drug registration approval, and GMP certification from the State Food and Drug Administration in China and started being using for exudative AMD treatment. It functions by competitively inhibiting the binding of VEGF with its receptor and prevents the activation of VEGFR by acting on multiple targets, thereby providing a new approach to the treatment of neovascular AMD. Many studies on aflibercept have shown its promise as an efficient drug for the treatment of neovascular AMD. In contrast, there are few reports on the efficacy of conbercept for neovascular AMD. Comparisons between conbercept and ranibizumab will not only determine the value of conbercept, but can also be referenced to aflibercept, as comparisons between aflibercept and ranibizumab have done.13, 14, 15
In this paper, we report the results from a comparison between conbercept and ranibizumab in the treatment of AMD using treat-and-extend (TREX) protocol. We hypothesized that conbercept would be at least as effective as ranibizumab in the treatment of AMD. In a single-arm analysis, Abedi et al16 were the first to report the results of a prospective TREX protocol of ranibizumab or bevacizumab anti-VEGF treatments. In their investigation, 120 consecutive patients with treatment-naive neovascular AMD had excellent visual outcomes reported, with fewer injections and clinic visits compared with monthly treatments. In another AMD study, visual and anatomic outcomes appear similar to those with fixed monthly dosing of ranibizumab.14 The trial reported in this article directly compares the efficacy and safety of conbercept with ranibizumab using a TREX approach to neovascular AMD management. A TREX approach to neovascular AMD is consistent with individualized management, while simultaneously minimizing treatment burden.
Materials and methods
Study patients
All patients signed an informed consent. This study adheres to the tenets of the declaration of Helsinki, and was approved by four Committees for Medical and Health Research Ethics (the First Hospital of Qiqihar, the Second Affiliated Hospital of Harbin Medical University, the Third Affiliated Hospital of Qiqihar Medical University and Peking Union Medical College Hospital). Between May 2014 and May 2015, 180 patients who received intravitreal injections of either conbercept or ranibizumab were collected from these four centers. Patients were examined at four ophthalmological centers in China as part of a multicenter study. Each patient was provided the names of the two drugs but no other drug information including biological contents and reported or known treatment results. Patients then selected a drug at the onset of treatment. Eligibility criteria included: age 51–85 years, and previously untreated active neovascular AMD in one eye. The baseline mean values of best-corrected visual acuity (BCVA) were 52.1 and 50.4 letters in conbercept group and ranibizumab group, respectively, as determined by protocol trial lens refraction; absence of other ocular diseases determined by examination using a tonometer, slit lamp biomicroscope and ophthalmoscope; lack of polypoidal choroidal vasculopathy as determined by indocyanine green angiography (ICGA); and the total area of the subretinal hemorrhage and fibrosis comprised less than 50% of the total lesion. Visual acuity was tested using Early Treatment Diabetic Retinopathy Study (ETDRS) charts at 4 m. The diagnosis of neovascular AMD was confirmed by choroidal neovascular leakage on fluorescein fundus angiography (FFA) and intraretinal or subretinal fluid as determined by optical coherence tomography (OCT). The mean central retinal thickness (CRT) was defined as the sum of the thickness of the neurosensory retina and the height of the subretinal fluid. Retinal pigment epithelial detachments were not included in the measurements. In 2016, the data for 1-year follow-up from 90 patients treated with each drug were collected and analyzed.
Study design
The patients received intravitreal injections of either conbercept 0.5 mg (0.05 ml) or ranibizumab 0.5 mg (0.05 ml) following a TREX protocol. Intravitreal injections were all completed by experienced ophthalmologists. Both drugs were acquired commercially, and batch numbers for all vials used in the study were registered. Sterile techniques were used for every injection. Prophylactic peri-intravitreal injection topical ophthalmic antibiotics were not deemed necessary and therefore were not used. Topical anesthesics were used (0.4% oxybuprocaine hydrochloride eye drops; Santen Pharmaceutical Co., Ltd, Osaka, Japan). The periocular skin, eyelids, and eyelashes were disinfected with 10% povidone–iodine swabs, and 5% povidone–iodine ophthalmic solution was applied to the ocular surface. Intraocular pressure (IOP) was measured within 1 h after injection. Increased IOP was defined as an intraocular pressure >25 mm Hg appearing within 24 h after injections. If IOP increased, subjects were monitored until intraocular pressure measured 25 mm Hg or less.
Applying TREX management, beginning at the third monthly treatment the interval between treatments was individually tailored based upon the exudative disease activity of each patient. Although patients were examined monthly, patients were treated no more frequently than every 4 weeks and no less frequently than every 12 weeks. At each visit, TREX patients were classified as having an active or inactive CNV lesion. Patients with active macula were then treated monthly until an inactive CNV lesion was achieved. An inactive CNV lesion was achieved upon resolution of intraretinal and subretinal fluid during the OCT examination and upon resolution of subretinal and intraretinal hemorrhage related to exudative AMD, as determined by fundus examination.17 If there were no signs of active neovascular disease, the period to the next treatment was extended by 2 weeks, up to a maximum interval of 12 weeks. A fluctuation of BCVA was not regarded as the criterion for the recurrence of disease. FFA was allowed to aid in re-treatment decisions. If clinical examination showed any sign of recurrence, the treatment interval was shortened by 2 weeks, until the disease was considered to be inactive. With the goal of avoiding multiple recurrences, the interval extension was then restarted, with the maximum final interval being 2 weeks less than the period when the previous recurrence was observed.5
Outcome measures
The primary outcome was BCVA, as measured using the ETDRS chart at each visit during a 1-year period. BCVA improvement was determined by protocol trial lens refraction for all the patients. The BCVA was measured beginning at the first visit and once a month afterward up to 12 months. Secondary outcomes included the number of injections, injection intervals, CRT, leakage of CNV, adverse events, and operative complications. Data were recorded and collected at four hospitals and then sent to the First Hospital of Qiqihar where the final analyses were conducted.
Statistical analysis
The margin of clinical non-inferiority was defined as five letters on the ETDRS visual acuity chart. Statistical analysis of the primary outcome variable, the mean change in BCVA from baseline to 1-year follow-up, was performed on data from the per protocol population (patients attending the 1-year visit). The mean scores of the primary outcome variables in both treatment groups were compared using the independent samples t-test. The same statistical procedure was applied when analyzing the data according to the intention-to-treat principle, using multiple imputation to replace missing observations at 1-year follow-up.
Statistical analysis of secondary outcomes was performed only on data from the per protocol population, except for CRT, for which multiple imputation was also applied. Continuous variables were presented as mean±standard deviation and compared using the independent samples t-test. Categorical variables were presented as percentages and compared between the two treatment groups using the χ2 test. A significance level of 5% was used throughout. All analyses were performed using SPSS software (version 18; SPSS Inc., Chicago, IL, USA) or the Student’s t-test.
Results
Patients and treatments
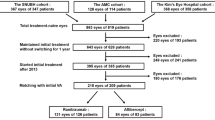
A total of 180 patients were included in the treatment and safety analysis, and the 1-year visit was completed by 168 (93.3%) patients. Of the 12 (6.7%) patients who discontinued treatment before the 1-year visit, among them three (1.7%) were diagnosed with other serious diseases. The three diseases were a heart failure and a hematencephalon in the conbercept group and a lung cancer in the ranibizumab group. Seven (3.9%) withdrew at their own request for economic and personal reasons (four in the conbercept group and three in the ranibizumab group). Two (1.1%) patients (one from each group) were excluded after serious retinal and vitreous hemorrhages a few days after inclusion. These were diagnosed by lack of fundus reflex, no view of the fundus, and blood was not absorbed. Further investigation was not conducted for these two patients, although stretches because of the contraction of neovascular membrane was suspected to be the cause of the vitreous hemorrhage. One case was treated with traditional Chinese medicine, and the other was treated by surgery after exit (Figure 1). There were no substantial differences between the groups regarding age, sex, IOP, BCVA, and CRT (Table 1). None of the patients had received prior similar treatment.
BCVA
At the end of 1 year, the visual acuity of the two groups was significantly improved after following a TREX protocol, and there was no significant difference between conbercept and ranibizumab cohorts. For all the patients who completed the 1-year observation, the mean BCVA improved by 12.7±7.770 and 12.3±7.269 letters in the conbercept and ranibizumab cohorts, respectively (P=0.624). The confidence interval (CI) was well within the stipulated non-inferiority limit of five letters. The intention-to-treat analysis demonstrated similar results, with a mean increase of 12.4 letters for conbercept and 12.1 for ranibizumab (P=0.813). BCVA improved by 15 or more letters in 19 eyes (22.9%) in the conbercept cohort and in 18 eyes (21.2%) in the ranibizumab cohort. Vision did not improve nor diminish in five eyes (6%) in the conbercept cohort and seven eyes (8.2%) in the ranibizumab cohort. In those eyes with no change in FFA, recurrent exudative activity and scar formation constituted the largest decrease in BCVA. The proportion did not differ between the treatment groups (P=0.619 and P=0.467, conbercept and ranibizumab, respectively). The BCVA was improved in 32 eyes (38.6%) and 35 eyes (41.2%) in the conbercept and ranibizumab cohorts after the first injections. The mean increases were 5.9 letters in the conbercept and 5.7 in the ranibizumab cohorts. The increase in BCVA was the most significant at the end of the third consecutive injection, with a mean increase of 10.1 letters for conbercept and 9.9 for ranibizumab cohorts (Figure 2a).
BCVA and treatment interval between conbercept and ranibizumab groups over 1 year. (a) The mean change in BCVA from baseline is indicated by the number of letters read on the ETDRS chart. BCVA gradually increased with treatment in both groups. The increases of BCVA were the most significant at the end of third month, with a mean of 10.1 letters for conbercept and 9.9 for ranibizumab cohorts. At the end of 1 year, the mean BCVA was improved by 12.7 and 12.3 letters in the conbercept and ranibizumab cohorts, respectively (P=0.624). (b) Mean treatment interval at 1 year. At 1 year after the start of treatment, the mean number of injections was 7.4 injections in the conbercept group and 8.7 injections in the ranibizumab group (P<0.001). There was a longer treatment interval distribution with a 12-week interval in the conbercept group, and a larger treatment interval distribution within 4 and 6 weeks in the ranibizumab group.
Injection numbers and treatment intervals
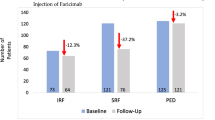
During the first year, the mean number of injections displayed a significant difference between the two groups with 7.4 (range, 6–11) injections for conbercept and 8.7 (range, 7–13) injections for ranibizumab (P<0.001) according to TREX management. At the end of the 1-year follow-up, the treatment intervals for conbercept group were 4 weeks in 24.1% (20 eyes), 6 weeks in 13.3% (11 eyes), 8 weeks in 8.4% (7 eyes), 10 weeks in 19.3% (16 eyes), and 12 weeks in 34.9% (29 eyes) of patients. In contrast, the ranibizumab group interval was 4 weeks in 31.8% (27 eyes), 6 weeks in 17.6% (15 eyes), 8 weeks in 10.6% (9 eyes), 10 weeks in 15.3% (13 eyes), and 12 weeks in 24.7% (21 eyes) of patients. Observation shows that there was a larger treatment interval distribution with a 12-week interval in the conbercept group, and more treatment interval distribution within 4 and 6 weeks in the ranibizumab group. The difference in the distribution of injection intervals was statistically significant between the two groups (P=0.011; Figure 2b).
CRT
At the end of the 1-year follow-up, the average CRT on OCT images was 215.3±42.5 μm in the conbercept group and 220.7±36.8 μm in the ranibizumab group after treatment. Both groups were significantly decreased compared with before treatment (406.8±47.1 and 408.5±52.4 μm for conbercept and ranibizumab, respectively; P<0.001). The intention-to-treat analysis was concordant. There was no significant difference in measured CRT, with a mean decrease of 191.5 μm for conbercept and 187.8 μm for ranibizumab (P=0.773) (Figure 3A).
Secondary outcomes in conbercept and ranibizumab groups over 1 year. (A) Mean change in CRT at 1 year after the start of treatment; the mean CRT was 215.3±42.5 μm in the conbercept group and 220.7±36.8 μm in the ranibizumab group. The decrease was most significant in the first month, then gradually tended to be stable in both treatment groups. (B) Changes in FFA, ICGA and OCT in the conbercept group at 1 year. Images showing clinical outcomes in fundus photography (a), FFA (b), ICGA (c) and OCT (d) before treatment. There was a large area of CNV in the macular area, accompanied by interretinal and subretinal hemorrhage, exudation and macular edema. Images showing clinical outcomes in fundus photography (e), FFA (f), ICGA (g) and OCT (h) after 12 months of treatment with conbercept. The leakage of CNV and activity of exudation were obviously improved, and the macular area tended to be dry.
Leakage of CNV
FFA and ICGA showed complete closure of CNV with 44 eyes (53%) in the conbercept group and 47 eyes (55.3%) in the ranibizumab group (P=0.589); partial closure with 31 eyes (37.3%) in the conbercept group and 29 eyes (34.1%) in the ranibizumab group (P=0.426); no change and recurrent exudative activity for eight eyes (9.6%) in the conbercept group and nine eyes (10.6%) in the ranibizumab group (P=0.547). Therefore, there was no statistical difference in the rate and degree of CNV recovery between these two groups (Figure 3B).
Adverse events
Table 2 summarizes the data of adverse events. One patient was excluded from each group after a serious retinal and vitreous hemorrhage a few days after inclusion, and corresponding treatments were given. IOP increased in four eyes (4.8%) in the conbercept group and five eyes (5.9%) in the ranibizumab group (P=0.823) after injection. One patient was given anterior chamber tap, and others were treated with IOP-lowering drugs. All of them decreased to normal ranges within 1 week. During the study, there were no cases of endophthalmitis or intraocular inflammation. Several incidence of diabetes mellitus and hypertension before and after treatment was found. However, there were no significant differences between the conbercept group and the ranibizumab group.
Discussion
At present, the clinical application of anti-VEGF drugs usually consists of monoclonal antibodies that function by blocking VEGF-A, a single target molecule. While they are effective, the drugs ranibizumab and bevacizumab are expensive and require multiple intraocular injections. Conbercept, a new anti-VEGF drug, independently developed in China, has successfully demonstrated efficacious results. The potential efficacy of ranibizumab and conbercept on neovascular AMD has been reported separately by our group and others in previous studies.18, 19, 20, 21 According to the phase I clinical trial of conbercept, patients with neovascular AMD given a single intravitreal injection of 3 mg of conbercept had an average increase in visual acuity of 19.6 letters after 42 days, with 57% of the subjects increasing by 15 letters or more.18 Our study is the first controlled study in which there is a direct comparison of conbercept with another widely used anti-VEGF drug. In our multicenter retrospective clinical study, patients treated with both conbercept and ranibizumab received satisfactory increases in BCVA at 1 year after implementing a TREX protocol. There was no significant difference in BCVA between the groups, demonstrating that the two drugs have equivalent effects on the regression of the neovascular component of AMD. Specifically, the mean increase in BCVA was 12.7 letters in the conbercept group and 12.3 letters in the ranibizumab group. Our study indicated that BCVA of most patients increased the most after the third consecutive injection, suggesting that visual acuity achieves the greatest improvement after three consecutive monthly injections. After the first three months, BCVA was stable or slowly increased.
The TREX approach has been used as the treatment method for neovascular AMD in USA and some other countries. Management using a TREX strategy significantly reduces the burden of care and cost of care delivery,19 and is used by more than 66% of retina specialists affiliated with the American Society of Retina Specialists in the United States.20 Our study indicated that it is sufficient to schedule follow-up visits based on treatment following TREX strategy after three monthly injections that begin the treatment.1 Our previous study using conbercept also indicated that less frequent dosing within the first three months can result in lesser optimal visual gains.21 Similar results using a TREX strategy have been elegantly illustrated in a study involving 1011 neovascular AMD patients from Australia and New Zealand who were managed with a TREX approach.22 In contrast to the monthly visits of a PRN (Pro re nata) protocol, with four or fewer treatments after the first three months, the current TREX protocol resulted in fewer office visits, less associated travel, and reduced cost burdens for patients. Similar results were also reported by the Lucentis Compared to Avastin Study (LUCAS) project, in which 441 patients in Norway were randomized to ranibizumab or bevacizumab treatment with a maximum extension interval of 12 weeks.5
Our conbercept and ranibizumab cohorts had no significant difference in baseline parameters. However, a statistically significant difference between the two groups was found in the injection intervals. At the end of 1 year, the mean number of injections was 7.4 for the conbercept and 8.7 for the ranibizumab cohorts. There was a peak treatment interval of 12 weeks in the conbercept group, while there was a peak of treatment interval distribution of 4–6 weeks in the ranibizumab group. Considering that conbercept is a VEGFR fusion protein, which is a natural conjugator of multiple targets of VEGFR, it is reasonable to speculate that it would have a longer duration of action.
In our TREX strategy, interval adjustment was based on the method of the LUCAS study.5 Thus, treatment intervals were lengthened progressively by 2 weeks until recurrent exudative disease was identified, at which point the interval was shortened by 2-week increments until a dry macula was re-established.5 In the case of patients with recurrence of disease, the treatment intervals were shortened by more than 2 weeks, while in the case of patients with scar formations, treatment intervals were lengthened by more than 2 weeks.23 Emphasizing this concept, intraocular levels of VEGF can vary among patients with phenotypically similar disease states.24 Therefore, treatment tailored according to individual clinical response and possibly genotype may be the most suitable approach for the clinical application of conbercept in AMD treatment.
The CRT in both of treatment groups was significantly decreased by treatment. There was a slightly more CRT improvement in the conbercept group than that in the ranibizumab group; however, this was not statistically significant. FFA showed less CNV complete closure in the conbercept group than that in the ranibizumab group. Partial closure was greater in the conbercept group than in the ranibizumab group; no change and recurrent exudative activity in the conbercept group were less than that in the ranibizumab group. In both groups, most of the patients with poor visual acuity were associated with recurrence or scarring in macular region.
Regarding adverse events, each treatment group had one patient excluded after a serious retinal and vitreous hemorrhage a few days after inclusion (Table 2). The safety and toxicity of conbercept has been studied in a phase 2 study.25 In our study, the membrane contraction of retinal neovascularization was suspected as the possible reason for serious hemorrhages in these two patients. Accordingly, treatment was given to these two patients. In addition, the IOP of several patients increased slightly (Table 2), but returned to normal within a week. A general reaction to intraocular injection is the possible reason for the increased IOP in these patients. Several new cases of hypertension and diabetes in both groups were identified during the 1-year follow-up. However, there were no statistically significant differences between the conbercept and ranibizumab groups for any of the adverse events. At the end of 1 year, there were no cases of endophthalmitis or intraocular inflammation in either cohort. However, additional long-term observation may be necessary.
The strength of our study is the overall comparison of the efficacy and safety of these two drugs, a VEGFR fusion protein and a VEGF monoclonal antibody, for the treatment of AMD. The collection of the patient data for the study was not based on any of the disease status and treatment results. The weakness of the study is the relatively short following-up period. Some patients may need the treatments for 2 years and even longer. Some adverse events, such as cardiovascular, cerebral vascular, and systemic diseases could occur in an extended period after the treatment. Finally, our study is not a randomized double blind design.
In conclusion, application of anti-VEGF drugs in the treatment of AMD is currently the main trend of treatment. This study confirmed that conbercept and ranibizumab had equivalent effects in visual gains and reduction of CRT at 1 year when administered according to a TREX protocol. However, there was a statistically significant difference between the drugs regarding the length of treatment intervals. In the conbercept group, more patients had reached longer treatment intervals and were offered the opportunity to reduce the treatment burden. However, because its application in clinical time is short, the long-term curative effect and the systemic complications have not been fully affirmed. It may be necessary for future thorough clinical research to compare its efficacy with drugs of similar structures such as Elyea, which is commercially used in the USA, but not in China. In addition, personalized treatment management needs to be more thoroughly explored.

References
Eye Diseases Prevalence Research Group. Causes and prevalence of visual impairment among adults in the United States. Arch Ophthalmol 2004; 122: 477–485.
Ye H, Zhang Q, Liu X, Cai X, Yu W, Yu S et al. Prevalence of age-related macular degeneration in an elderly urban chinese population in China: the Jiangning Eye Study. Invest Ophthalmol Vis Sci 2014; 55 (10): 6374–6380.
Souied E, Kaplan J, Coscas G, Soubrane G . Age-related macular degeneration and genetics J. J Fr Ophtalmol 2001; 24 (8): 875–885.
Vedula SS, Krzystolik MG . Antiangiogenic therapy with anti-vascular endothelial growth factor modalities for neovascular age-related macular degeneration. Cochrane Database Syst Rev 2008; 16: CD005139.
Berg K, Pedersen TR, Sandvik L, Bragadóttir R . Comparison of ranibizumab and bevacizumab for neovascular age-related macular degeneration according to LUCAS treat-and-extend protocol. Ophthalmology 2015; 122: 146–152.
Rosenfeld PJ, Fung AE, Puliafito CA . Optical coherence tomography findings after an intravitreal injection of bevacizumab (Avastin) for macular edema from central retinal vein occlusion. Ophthalmic Surg Lasers Imaging 2005; 36: 336–339.
Witmer AN, Vrensen GFJM, Van Noorden CJF, Schlingemann R . Vascular endothelial growth factors and angiogenesis in eye disease. Prog Retin Eye Res 2003; 22: 1–29.
Brown DM, Kaiser PK, Michels M, Soubrane G, Heier JS, Kim RY et al. Ranibizumab versus verteporfin for neovascular age-related macular degeneration. N Engl J Med 2006; 355: 1432–1444.
Heier JS, Brown DM, Chong V, Korobelnik JF, Kaiser PK, Nguyen QD et al. Intravitreal aflibercept (VEGF Trap-Eye) in wet age-related macular degeneration. Ophthalmology 2012; 119: 2537–2548.
Martin DF, Maguire MG, Fine SL, Ying G, Jaffe GJ, Grunwald JE et al. Ranibizumab and bevacizumab for treatment of neovascular age-related macular degeneration: two-year results. Ophthalmology 2012; 119: 1388–1398.
Mitchell EP . Targeted therapy for metastatic colorectal cancer: role of aflibercept. Clin Colorectal Cancer 2013; 12 (2): 73–85.
Lu X, Sun X . Profile of conbercept in the treatment of neovascular age-related macular degeneration. Drug Des Dev Ther 2015; 9: 2311–2320.
Chaikitmongkol V, Bressler NM . Dramatic resolution of choroidal neovascular abnormalities after single aflibercept injection following years of ranibizumab use. JAMA Ophthalmol 2013; 131 (2): 260–262.
Kumar N, Marsiglia M, Mrejen S, Fung AT, Slakter J, Sorenson J et al. Visual and anatomical outcomes of intravitreal aflibercept in eyes with persistent subfoveal fluid despite previous treatments with ranibizumab in patients with neovascular age-related macular degeneration. Retina 2013; 33 (8): 1605–1612.
Avery RL, Castellarin AA, Steinle NC, Dhoot DS, Pieramici DJ, See R et al. Systemic pharmacokinetics following intravitreal injections of ranibizumab, bevacizumab or aflibercept in patients with neovascular AMD. Br J Ophthalmol 2014; 98 (12): 1636–1641.
Abedi F, Wickremasinghe SS, Islam AF, Guymer RH . Anti-VEGF treatment in neovascular age-related macular degeneration: a treat-and-extend protocol over 2 years. Retina 2014; 34: 1531–1538.
Wykoff CC, Croft DE, Brown DM, Wang R, Payne JF, Clark L et al. Prospective trial of treat-and-extend versus monthly dosing for neovascular age-related macular degeneration: TREX-AMD 1-year results. Ophthalmology 2015; 122: 2514–2522.
Zhang M, Zhang J, Yan M, Luo D, Zhu W, Kaiser PK et al. A phase 1 study of KH902, a vascular endothelial growth factor receptor decoy, for exudative age-related macular degeneration. J Ophthalmol 2011; 118 (4): 672–678.
Gupta OP, Shienbaum G, Patel AH, Fecarotta C, Kaiser RS, Regillo CD . A treat and extend regimen using ranibizumab for neovascular age-related macular degeneration clinical and economic impact. Ophthalmology 2010; 117: 2134–2140.
American Society of Retinal Specialists PAT Survey. 2015. Available at: https://www.asrs.org/content/documents/2015_global_trends_in_retina_survey_-_for_website.pdf(Accessed 11 July 2016.)
Lu H, Cui J, Dong H, Luo B, Xiu W, Li H . Clinical observation of a new anti-VEGF drugs conbercept for wet age-related macular degeneration. Chin J Ophthalmol 2015; 51 (11): 818–821.
Arnold JJ, Campain A, Barthelmes D, Simpson JM, Guymer RH, Hunyor AP et al. Two-year outcomes of ‘treat and extend’ intravitreal therapy for neovascular age-related macular degeneration. Ophthalmology 2015; 122: 1212–1219.
Brown DM, Tuomi L, Shapiro H . Anatomical measures as predictors of visual outcomes in ranibizumab-treated eyes with neovascular age-related macular degeneration. Retina 2013; 33: 23–34.
Muether PS, Hermann MM, Viebahn U, Kirchhof B, Fauser S . Vascular endothelial growth factor in patients with exudative age-related macular degeneration treated with ranibizumab. Ophthalmology 2012; 119: 2082–2086.
Li X, Xu G, Wang Y, Xu X, Liu X, Tang S et al. Safety and efficacy of conbercept in neovascular age-related macular degeneration: results from a 12-month randomized phase 2 study: AURORA study. Ophthalmology 2014; 121 (9): 1740–1747.
Acknowledgements
This work was partially supported by funding from The First Hospital of Qiqihar City, Heilongjiang province, PR China; the National Natural Science Foundation of China (Project 81372996 to YJ), PR China; an unrestricted grant from Research to Prevent Blindness (New York, NY); a merit grant (I01 BX000671 to WG) from the U.S. Department of Veterans Affairs and the Veterans Administration Medical Center in Memphis, TN. The funding organizations had no role in the design or conduct of this research.
Author contributions
Conceptualization: JC, DS, HC and WG; formal analysis: JC, WG, MMJ and SC; funding acquisition: JC, MMJ, HC, YJ, WG, DS, RD and LX; investigation: JC, HL, MMJ, YJ, DS, RD, LX, LW and BJ; methodology: HC, YJ, WG, DS, RD and LX; project administration: LW, DW and HC; resources: LW, DW, HC, MMJ, WG and SC; supervision: LW, DS, DW and HC; validation: JC, DS, MMJ, SC and WG; writing original draft JC, WG and MMJ; and writing review & editing: all authors.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Rights and permissions
This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in the credit line; if the material is not included under the Creative Commons license, users will need to obtain permission from the license holder to reproduce the material. To view a copy of this license, visit http://creativecommons.org/licenses/by-nc-nd/4.0/
About this article
Cite this article
Cui, J., Sun, D., Lu, H. et al. Comparison of effectiveness and safety between conbercept and ranibizumab for treatment of neovascular age-related macular degeneration. A retrospective case-controlled non-inferiority multiple center study. Eye 32, 391–399 (2018). https://doi.org/10.1038/eye.2017.187
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/eye.2017.187
This article is cited by
-
Disease stability and extended dosing under anti-VEGF treatment of exudative age-related macular degeneration (AMD) — a meta-analysis
Graefe's Archive for Clinical and Experimental Ophthalmology (2021)
-
Conbercept for patients with age-related macular degeneration: a systematic review
BMC Ophthalmology (2018)
-
Letter to the Editor
Eye (2018)