Abstract
Purpose
To assess contrast sensitivity (CS) and to elucidate the factors associated with CS among subjects with type 2 diabetes in a cross-sectional population-based study.
Patients and methods
Subjects were recruited from a follow-up cohort, Sankara Nethralaya Diabetic Retinopathy Epidemiology and Molecular genetics Study (SN-DREAMS II). Of 958 subjects who were followed up in SN-DREAMS II, a subset of 653 subjects was included in the analysis. All subjects underwent a comprehensive eye examination, which included CS assessment using the Pelli–Robson chart. The cross-sectional association between CS and independent variables was assessed using stepwise linear regression analysis. A P-value of <0.05 was considered statistically significant.
Results
The mean age of the study sample was 58.7±9.41 (44–87) years. Mean CS of the study sample was 1.32±0.20 (range: 0–1.65) log units. CS was negatively and significantly correlated with age, duration of diabetes, hemoglobin level, vibration perception threshold (VPT) value, albuminuria, best corrected visual acuity (BCVA), refractive error, total error score (TEM) of FM 100 hue test, and mean retinal sensitivity. In multiple regression analysis, after adjusting for all the related factors, CS was significantly associated with BCVA (β=−0.575; P<0.001), VPT (β=−0.003; P=0.010), severity of cataract (β=−0.018; P=0.032), diabetic retinopathy (β=−0.016; P=0.019), and age (β=−0.002; P=0.029). These factors explained about 29.3% of the variation in CS.
Conclusion
Among the factors evaluated, differences in BCVA were associated with the largest predicted differences in CS. This association of CS with visual acuity highlights the important role of visual assessment in type 2 diabetes.
Similar content being viewed by others
Introduction
Retinopathy, the most frequent microvascular complication of diabetes, remains a leading cause of acquired blindness among working age adults.1, 2 The prevalence of diabetic retinopathy (DR) is reported to be 18% among subjects with diabetes mellitus in the urban population in India.3 Earlier studies have shown that changes in the ganglion layer, inner retinal neurons, and peripheral network may be present in the asymptomatic stages of DR.4, 5 There is evidence of thinning of the inner retina in patients with minimal retinopathy, caused primarily by a thinning of the ganglion cell layer in the pericentral area of the macula, and secondarily by thinning of the retinal nerve fiber layer more peripherally in the macula, because of axonal loss from the central ganglion cells.6
The characteristic ‘spatial tuning’ of ganglion cell receptive fields is reflected in peaked contrast sensitivity functions.7 The evaluation of contrast sensitivity in people with diabetes is important, as the reduction of contrast sensitivity can lead to difficulties in variety of tasks of daily living like stair descent, stair ascent, inserting a key in a lock, dialing a rotary telephone, face recognition and reading speed, and so on.8, 9, 10, 11 Various studies have evaluated CS in diabetes with and without DR.12, 13, 14, 15, 16, 17, 18 Factors reportedly associated with abnormal CS in diabetes include age,12, 13 visual acuity,14 duration of diabetes,13 glycosylated hemoglobin (HbA1c),14 and presence of DR.15, 16 The reports are inconsistent as the studies varied in their methodology, that is, different techniques were used to assess the contrast sensitivity, study population was different as few studies included type 1 and others type 2 diabetes, few considered subjects without DR, and others with various stages of DR. And all the studies have not explored various systemic and ocular factors and their relation with the contrast sensitivity. The relationship between abnormal CS and vascular and metabolic abnormalities in diabetes has not been extensively studied in the Indian population.
The aim of the present study was to examine CS in a population-based cohort of subjects with type 2 diabetes and assess its correlation with systemic, biochemical, and ocular characteristics.
Patients and methods
Sankara Nethralaya Diabetic Retinopathy Epidemiology and Molecular Genetic Study (SN-DREAMS II) was a follow-up study of SN-DREAMS I.19 Among the 958 subjects followed in SN-DREAMS II, 653 subjects who have undergone contrast sensitivity test were included for the current analysis. Contrast sensitivity was not assessed in 146 subjects as they were illiterate. The study was approved by the organization’s Institutional Review Board, and informed consent was obtained from the subjects in accordance with the Declaration of Helsinki. One eye of each subject was analyzed. If the subject did not have DR in either eye, the right eye was selected; if DR changes were seen in one eye, that eye was included; and if both eyes had evidence of DR, the eye with more severe DR grade was considered for analysis.
Demographic data and detailed medical and ocular history were obtained from each subject. The various factors included in the current study were further classified as demographic factors and systemic factors, such as duration of diabetes, HbA1c level, hypertension, hemoglobin, nephropathy, neuropathy, and lipid profile (serum total cholesterol, triglycerides, high-density lipoprotein, and low-density lipoprotein). Ocular parameters included are BCVA, refractive error, color vision, cataract status, DR status, central retinal thickness (CRT), photoreceptor layer thickness, and mean retinal sensitivity.
Hypertension was defined as systolic blood pressure ≥140 mm Hg or diastolic blood pressure ≥90 mm Hg, or a history of using antihypertensive medications. Biochemical investigations (total serum cholesterol, high-density lipoproteins, serum triglycerides, hemoglobin, and HbA1c) were conducted at the base hospital in a fasting state. The low-density lipoprotein was calculated using the modified Friedewald formula for the Indian population.20 Glycemic control was categorized as normal (HbA1c<5.6%), good to fair (HbA1c 5.6–8.0%), or poor (HbA1c>8.1%).21 Anemia was defined as a hemoglobin concentration of <13 g/dl in men or <12 g/dl in women.22 The patient was considered normoalbuminuric if albumin creatinine ratio (ACR) was <0 mg/g, microalbuminuric if the ACR was between 30 and 300 mg/g, and macroalbuminuric if the ACR was above 300 mg/g.23 Diabetic nephropathy was defined as present if the ACR is ≥30 mg/g.
Quantitative assessment of vibration perception threshold (VPT) is a widely applied tool in the screening for diabetic sensory neuropathy, particularly in epidemiological studies. Diabetic neuropathy was assessed by measuring VPT using a sensitometer (Sensitometer-VPT, Dhansai Laboratory, Mumbai, India). The VPT was measured by a single observer by placing a biothesiometer probe perpendicular to the distal plantar surface of the great toe of both legs. The VPT was measured at a voltage level when patient felt the first vibration sensation. The mean VPT measure of three readings for each leg was considered for the analysis. Diabetic neuropathy was considered present if the VPT value was >20 V.24
All subjects underwent a comprehensive ophthalmic examination. Contrast sensitivity was assessed using a Pelli–Robson chart25 at 100 cd/m2 measured at 1 m distance with the best corrected refractive error based on the subjective refraction performed on the same day. The logarithmic CS value of the last triplet of which at least two letters are correctly seen is marked as the result. Color discrimination was assessed using the Farnsworth–Munsell 100 (FM 100) hue test under viewing booth illuminated designed by Zahiruddin et al,26 and the total error score (TES) was calculated. Lens opacity was graded according to the Lens Opacity Classification System (LOCS) III.27 Cataract was graded as ‘no cataract =0, NC=1, CC=2, PSC=3, Mixed=4’. DR was graded clinically using Klein’s classification (Modified Early Treatment Diabetic Retinopathy Study scales).28 Severity of DR was coded as ‘no DR=0, mild non-proliferative diabetic retinopathy (NPDR)= 1, moderate NPDR=2, severe NPDR=3, proliferative DR=4, clinically significant macular edema=5’ for the purpose of analysis. After pupillary dilatation retinal photographs were obtained with the Carl Zeiss FF 450 Plus IR Fundus Camera; all patients underwent 45°, 4-field stereoscopic digital photography (posterior, nasal, superior, and inferior poles). For those who showed evidence of any retinopathy, additional 30°, 7-field stereo digital pairs were obtained. All photographs were graded by two independent observers in a masked fashion; the grading agreement was high (k=0.83).
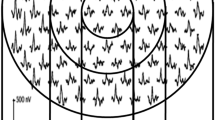
Mean retinal sensitivity was assessed using MP1 microperimeter (Nidek Technologies, Padova, Italy); CRT and photoreceptor layer thickness (PRL) were assessed using spectral domain optical coherence tomography (Copernicus, Poland).
Statistical analysis
Statistical analyses were performed using the statistical software SPSS for Windows, ver.15.0 (SPSS Science, Chicago, IL, USA). Duration of diabetes for newly diagnosed subjects with diabetes was considered as 0. Contrast sensitivity was represented as mean±SD. Both unadjusted and adjusted linear regression was performed for the variables with contrast sensitivity as a dependent variable. Stepwise linear regression was used for selection of variables for the multivariate model. The linearity assumption for variables included in the model as continuous factors was checked by visual inspection of scatter plots. A P-value of <0.05 was considered statistically significant.
Results
The mean age of the study sample was 58.7±9.41 (44–87) years and 384 of the subjects (58.8%) were male. Mean duration of diabetes mellitus was 8.94±6.24 (0–37) years and mean logMAR visual acuity of the study sample was 0.063±0.14 (−0.10–1.3) log units. Mean CS of the study sample was 1.32±0.20 (0–1.65) log units. Table 1 shows the distribution of CS among the demographic and systemic factors. CS was significantly reduced among the older age groups (P<0.001). CS was noted to be significantly lower among subjects with a duration of diabetes of more than 5 years (1.30±0.20 vs 1.36±0.19; P<0.001), subjects with anemia (1.29±0.20 vs 1.34±0.20; P=0.002), those with neuropathy (1.26±0.21 vs 1.34±0.19; P<0.001), those who were taking insulin (1.27±0.16 vs 1.33±0.20; P=0.047), and those with poor glycemic control (P=0.002).
Table 2 shows the distribution of CS among the ocular parameters. CS was significantly reduced in those with visual acuity worse than 0.00 logMAR (1.22±0.22 vs 1.37±0.17; P<0.001), those with refractive error (1.30±0.21 vs 1.35±0.18; P=0.003), and those with an abnormal color vision TES>100 (1.31±0.19 vs 1.36±0.14; P=0.047). Other ocular factors associated with a significantly reduced CS were the presence of cataract (P<0.001), presence of DR (P=0.003), and presence of sight-threatening DR (1.20±0.20 vs 1.30±0.19; P=0.007).
Factors associated with reduced CS in univariate analysis were increased age (β=−0.007, P<0.001), increased duration of diabetes (β=−0.004, P=0.002), increased VPT score, that is, diabetic neuropathy (β=−0.007, P<0.001), use of insulin (β=−0.06, P=0.047), poor BCVA (β=−0.695, P<0.001), increased severity of DR (β=−0.029, P<0.001), presence of cataract (β=−0.065, P<0.001), history of cataract surgery (β=−0.079, P<0.001), and increased TES on the FM 100 (β=−0.001, P<0.001). An increase in mean retinal sensitivity (β=0.021, P<0.001) and an increase in hemoglobin (β=0.012, P=0.002) were associated with increased CS.
Table 3 summarizes the coefficients when analyzed in a multiple linear regression model and quantifies the relationship between CS and the variables. With a 0.1 log unit worsening of BCVA, CS is reduced by 0.575 units. With an increase in VPT score and cataract severity on LOCS III, CS is decreased by 0.003 and 0.018 log units. We also found that a 1 grade increase in DR was associated with an average of 0.016 units decrease in CS. With every 1 year increase in age, CS is reduced by 0.002 units.
Following model predicts the CS based on both systemic and ocular parameters.
Predicted CS=1.594+(−0.575) BCVA+(−0.003) VPT score+(−0.018) cataract grading by LOCS III+(−0.016) stage of DR+(−0.002) age.
Discussion
In this population-based study we report that CS was significantly reduced in the presence of DR. The mean CS in our subjects with diabetes was 1.32±0.20 log units, which was <1.44±0.05 log units reported by Stavrou et al18 The difference could be because of the larger sample size in our study and that they included only subjects with early DR, whereas our study included subjects with various stages of DR, as well as subjects with diabetes but without DR. Mantyjarvi et al29 reported normal CS ranged from 1.68 to 1.84 log units in healthy individuals, which was high compared with our diabetic sample. We found an inverse association between CS and severity of DR consistent with previous reports.15, 16 However, these studies had several limitations, including the lack of adjustment of the various factors affecting CS, lack of demonstration of an established association among the risk factors and the CS. To the best of our knowledge this is the first study to assess various risk factors for CS in a population-based study in south India. The prevalence of DR in the study sample was 20.9%, and diabetic macular edema among subjects with DR was 16%. Prevalence of neuropathy was 26% and that of nephropathy was 25.39%. Among the diabetic microangiopathies, DR and neuropathy were associated with CS, which was consistent with earlier reports.15, 16, 18
Frisen30 reported that visual acuity is unaffected until 55% of all neuroretinal channels are affected. It has been well documented that contrast sensitivity may be compromised despite normal visual acuity among subjects with diabetes but no retinopathy.18 Vision in the low-contrast domain is as important as that in high-contrast domain. There are various tasks done by subjects with diabetes where they require good contrast sensitivity, one such task is checking of urine or blood sugar level. Few patients use contrast differences as the clue when lowered color discrimination disturbs the reading of the test results. As the contrast sensitivity and color vision are slowly impaired, subjects are unaware of their inability. Periodic evaluation of visual function even in the absence of retinopathy helps in early detection and management of the progression of disease.
Age is an established risk factor for reduced contrast sensitivity;12, 13 and this association was confirmed in our current study. The Salisbury Eye Evaluation (SEE) study evaluated the relations among various measures of visual function in older population and reported a strong relationship between contrast sensitivity and age.31 It has been reported that reduced contrast sensitivity is related to both optical and neural factors.12 Anemia has been reported to be associated with the development and progression of both micro- and macrovascular complications of diabetes.32 CS was positively correlated with hemoglobin levels in the present study. This may be due to the retinal vascular and visual defects caused by localized retinal tissue hypoxia in subjects with diabetes.33, 34 Studies have reported that hyperoxia significantly improved mean contrast sensitivity in subjects with diabetes.35 VPT values showed a significant negative correlation with CS (r=−0.238, P<0.001), which was in contrast to the study done by Moaven-Shahidi A et al,36 who reported that CS did not vary between subjects with and without neuropathy.
A statistically significant inverse correlation was found between CS and logMAR visual acuity (β=−0.575, P<0.001), consistent with the results reported by Misra et al14 (β=−0.427, P<0.001). The presence of cataract was significantly associated with reduced CS; consistent with the earlier reports.37, 38, 39 Studies reported that nuclear and cortical cataracts influence median and high-frequency CS; whereas PSC impacted low-frequency CS.40, 41, 42 In our study, in contrast, cataract types influenced CS at 1 cpd, which is a measure of from the Pelli–Robson test.
Mean retinal sensitivity was positively correlated with CS, but it showed no association on multiple regression after adjusting for all variables. No correlation was found between CS and CRT or PRL in this study. This could be explained by the early impact of diabetes on the inner retina,43 even though outer retina is also involved. Both histopathologic and imaging studies have reported that diabetes causes apoptosis of ganglion cells and their dysfunction is primarily responsible for the abnormal CS.6, 43, 44, 45 Assessment of inner retinal thickness and function of ganglion cells would have added more value for this study.
Presence of DR was significantly associated with abnormal CS, as reported by previous studies using various techniques. Using an oscilloscope to generate gratings, Howes et al37 found a systematic decrease in CS with severity of background DR. They also reported that background retinopathy produced abnormal CS at low and medium frequencies. In contrast, Sokol et al16 reported that no specific spatial frequency is selectively affected in DR. With a 1-step increase in severity of DR, CS was reduced significantly by 0.016 units. The disturbance of visual function may be linked to vascular damage and thus correlated to the degree of retinopathy.
The regression model explains about 29.3% of the variation in CS. The main purpose of the model was to explore the relative importance of ocular and systemic factors for the assessment of CS in type 2 diabetes sample. In conclusion, contrast sensitivity is impaired in DR. The changes in contrast sensitivity are related to both ocular and systemic characteristics in type 2 diabetes and not alone on severity of DR, as is clear from our study about various systemic and ocular risk factors associated with CS in subjects with diabetes. This is important as the reduction of contrast is associated with impaired performance of tasks in domains of mobility, activities of daily life, and visually intensive tasks.

References
Congdon N, O'Colmain B, Klaver CC, Klein R, Muñoz B, Friedman DS et al. Causes and prevalence of visual impairment among adults in the United States. Arch Ophthalmol 2004; 122: 477–485.
Patz A, Smith RE . The EDTRS and Diabetes 2000. Ophthalmology 1991; 98 (5 Suppl): 739–740.
Raman R, Rani PK, Reddi Rachepalle S, Gnanamoorthy P, Uthra S, Kumaramanickavel G et al. Prevalence of diabetic retinopathy in India: Sankara Nethralaya Diabetic Retinopathy Epidemiology and Molecular Genetics Study report 2. Ophthalmology 2009; 116: 311–318.
Arend O, Remky A, Evans D, Stuber R, Harris A . Contrast sensitivity loss is coupled with capillary dropout in patients with diabetes. Invest Ophthalmol Vis Sci 1997; 38: 1819–1824.
Gardner TW, Antonetti DA, Barber AJ, LaNoue KF, Levison SW . Diabetic retinopathy: more than meets the eye. Surv Ophthalmol 2002; 47: S253–S262.
van Dijk HW, Verbraak FD, Kok PH, Garvin MK, Sonka M, Lee K et al. Decreased retinal ganglion cell layer thickness in patients with type 1 diabetes. Invest Ophthalmol Vis Sci 2010; 51: 3660–3665.
Nelson Ralph Ganglion Cell Physiology by Ralph Nelson; Webvision, The Organization of the Retina and Visual System. Available at: http://webvision.med.utah.edu/book/part-ii-anatomy-and-physiology-of-the-retina/ganglion-cell-physiology/. Accessed on 17 June 2014.
Tran TH, Despretz P, Boucart M . Scene perception in age-related macular degeneration: the effect of contrast. Optom Vis Sci 2012; 89: 419–425.
Donoghue OA, Ryan H, Duggan E, Finucane C, Savva GM, Cronin H et al. Relationship between fear of falling and mobility varies with visual function among older adults. Geriatr Gerontol Int 2014; 14: 827–836.
West SK, Rubin GS, Broman AT, Muñoz B, Bandeen-Roche K, Turano K . How does visual impairment affect performance on tasks of everyday life? The SEE Project. Salisbury Eye Evaluation. Arch Ophthalmol 2002; 120: 774–780.
Ross JE, Bron AJ, Clarke DD . Contrast sensitivity and visual disability in chronic simple glaucoma. Br J Ophthalmol 1984; 68: 821–827.
Arundale K . An investigation into the variation of human contrast sensitivity with age and ocular pathology. Br J Ophthalmol 1978; 62: 213–215.
Liska V, Dostálek M . Are contrast sensitivity functions impaired in insulin dependent diabetics without diabetic retinopathy? Acta Medica (Hradec Kralove) 1999; 42: 133–138.
Misra S, Saxena S, Kishore P, Bhasker SK, Misra A, Meyer CH . Association of contrast sensitivity with LogMAR visual acuity and glycosylated hemoglobin in non-insulin dependent diabetes mellitus. J Ocul Biol Dis Infor 2010; 3: 60–63.
Ghafour IM, Foulds WS, Allan D, McClure E . Contrast sensitivity in diabetic subjects with and without retinopathy. Br J Ophthalmol 1982; 66: 492–495.
Sokol S, Moskowitz A, Skarf B, Evans R, Molitch M, Senior B . Contrast sensitivity in diabetics with and without background retinopathy. Arch Ophthalmol 1985; 103: 51–54.
Rodríguez-Galietero A, Montés-Micó R, Muñoz G, Albarrán-Diego C . Blue-light filtering intraocular lens in patients with diabetes: contrast sensitivity and chromatic discrimination. J Cataract Refract Surg 2005; 31: 2088–2092.
Stavrou EP, Wood JM . Letter contrast sensitivity changes in early diabetic retinopathy. Clin Exp Optom 2003; 86: 152–156.
Agarwal S, Raman R, Paul PG, Rani PK, Uthra S, Gayathree R et al. Sankara Nethralaya Diabetic Retinopathy Epidemiology and Molecular Genetic Study (SN-DREAMS 1): study design and research methodology. Ophthalmic Epidemiol 2005; 12: 143–153.
Anandaraja S, Narang R, Godeswar R, Laksmy R, Talwar KK . Low-density lipoprotein cholesterol estimation by a new formula in Indian population. Int J Cardiol 2005; 102: 117–120.
Expert Committee on the Diagnosis and Classification of Diabetes Mellitus. Report of the expert committee on the diagnosis and classification of diabetes mellitus. Diabetes Care 2003; 26: S5–20.
Bosman DR, Winkler AS, Marsden JT, Macdougall IC, Watkins PJ . Anemia with erythropoietin deficiency occurs early in diabetic nephropathy. Diabetes Care 2001; 24: 495–499.
Namperumalsamy P, Nirmalan PK, Ramasamy K . Developing a screening program to detect sight-threatening diabetic retinopathy in South India. Diabetes Care 2003; 26: 1831–1835.
Shaw JE, Gokal R, Hollis S, Boulton AJ . Does peripheral neuropathy invariably accompany nephropathy in type 1 diabetes mellitus? Diabetes Res Clin Pract 1998; 39: 55–61.
Pelli DG, Robson JG, Wilkins AJ . The design of a new letter chart for measuring contrast sensitivity. Clin Vis Sci 1988; 2: 187–189.
Zahiruddin K, Banu S, Dharmarajan R, Kulothungan V, Vijayan D, Raman R et al. Effect of illumination on colour vision testing with Farnsworth-Munsell 100 hue test: customized colour vision booth versus room illumination. Korean J Ophthalmol 2010; 24: 159–162.
Chylack Jr LT, Wolfe JK, Singer DM, Leske MC, Bullimore MA, Bailey IL et al. The Lens Opacities Classification System III. The Longitudinal Study of Cataract Study Group. Arch Ophthalmol 1993; 111: 831–836.
Klein R, Klein BE, Magli YL, Brothers RJ, Meuer SM, Moss SE et al. An alternative method of grading diabetic retinopathy. Ophthalmology 1986; 93: 1183–1187.
Mäntyjärvi M, Laitinen T . Normal values for the Pelli-Robson contrast sensitivity test. J Cataract Refract Surg 2001; 27: 261–266.
Frisen FM . A simple relationship between the probability distribution of visual acuity and the density of retinal output channels. Acta Ophthalmol 1976; 54: 437–444.
Rubin GS, West SK, Muñoz B, Bandeen-Roche K, Zeger S, Schein O et al. A comprehensive assessment of visual impairment in a population of older Americans. The SEE Study. Salisbury Eye Evaluation Project. Invest Ophthalmol Vis Sci 1997; 38: 557–568.
Thomas MC, MacIsaac RJ, Tsalamandris C, Power D, Jerums G . Unrecognized anemia in patients with diabetes: a cross-sectional survey. Diabetes Care 2003; 26: 1164–1169.
Grunwald JE, Riva CE, Brucker AJ, Sinclair SH, Petrig BL . Altered retinal vascular response to 100% oxygen breathing in diabetes mellitus. Ophthalmology 1984; 91: 1447–1452.
Fallon TJ, Maxwell DL, Kohner EM . Autoregulation of retinal blood flow in diabetic retinopathy measured by the blue light entoptic technique. Ophthalmology 1987; 94: 1410–1415.
Harris A, Arend O, Danis RP, Evans D, Wolf S, Martin BJ . Hyperoxia improves contrast sensitivity in early diabetic retinopathy. Br J Ophthalmol 1996; 80: 209–213.
Moaven-Shahidi A . Assessment of retinal structure and visual function in association with diabetic peripheral neuropathy. PhD thesis,. Queensland University of Technology, 2011 pages 288 Available at: http://eprints.qut.edu.au/46227/1/Ayda_Moaven-Shahidi_Thesis.pdf.
Howes SC, Caelli T, Mitchell P . Contrast sensitivity in diabetics with retinopathy and cataract. Aust J Ophthalmol 1982; 10: 173–178.
Cheng Y, Shi X, Cao XG, Li XX, Bao YZ . Correlation between contrast sensitivity and the lens opacities classification system III in age-related nuclear and cortical cataract. Chin Med J 2013; 126: 1430–1435.
Williamson TH, Strong NP, Sparrow J, Aggarwal RK, Harrad R . Contrast sensitivity and glare in cataract using the Pelli-Robson chart. Br J Ophthalmol 1992; 76: 719–722.
Chua BE, Mitchell P, Cumming RG . Effects of cataract type and location on visual function: the Blue Mountains Eye Study. Eye 2004; 18: 765–772.
Drews-Bankiewicz MA, Caruso RC, Datiles MB, Kaiser-Kupfer MI . Contrast sensitivity in patients with nuclear cataracts. Arch Ophthalmol 1992; 110: 953–959.
Elliott DB, Gilchrist J, Whitaker D . Contrast sensitivity and glare sensitivity changes with three types of cataract morphology: are these techniques necessary in a clinical evaluation of cataract? Ophthalmic Physiol Opt 1989; 9: 25–30.
Van Dijk HW, Kok PH, Garvin M, Sonka M, Devries JH, Michels RP et al. Selective loss of inner retinal layer thickness in type 1 diabetic patients with minimal diabetic retinopathy. Invest Ophthalmol Vis Sci 2009; 50: 3404–3409.
Abu-El-Asrar AM, Dralands L, Missotten L, Al-Jadaan IA, Geboes K . Expression of apoptosis markers in the retinas of human subjects with diabetes. Invest Ophthalmol Vis Sci 2004; 45: 2760–2766.
Takahashi H, Chihara E . Impact of diabetic retinopathy on quantitative retinal nerve fiber layer measurement and glaucoma screening. Invest Ophthalmol Vis Sci 2008; 49: 687–692.
Acknowledgements
The study was supported by Jamshetji Tata trust, Mumbai.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Rights and permissions
About this article
Cite this article
Gella, L., Raman, R., Pal, S. et al. Contrast sensitivity and its determinants in people with diabetes: SN-DREAMS-II, Report No 6. Eye 31, 460–466 (2017). https://doi.org/10.1038/eye.2016.252
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/eye.2016.252