Abstract
Macular edema (ME) may complicate anterior, intermediate, and posterior uveitis, which may be because of various infectious, neoplastic or autoimmune etiologies. BRB breakdown is involved in the pathogenesis of Uveitic ME (UME). Optical coherence tomography has become a standard tool to confirm the diagnosis of macular thickening, due to its non-invasive, reproducible, and sensitive features. Retinal fluorescein and indocyanine green angiography is helpful to study the macula and screen for associated vasculitis, detect ischemic areas and preretinal, prepapillary or choroidal neovascular complications, and it may provide information about the etiology and be needed to assess the therapeutic response. UME due to an infection or neoplastic infiltration may require a specific treatment. If it remains persistent or occurs in other etiologies, immunomodulatory treatments may be needed. Intravitreal, subconjunctival, or subtenon corticosteroids are widely used. Their local use is contraindicated in glaucoma patients and limited by their short-lasting action. In case of bilateral sight-threatening chronic posterior uveitis, systemic treatments are usually needed, and corticosteroids are used as the standard first-line therapy. In order to reduce the daily steroid dose, immunosuppressive or immunomodulatory agents may be added, some of them being now available intravitreally. Ongoing prospective studies are assessing biotherapies and immunomodulators to determine their safety and efficacy in this indication.
Similar content being viewed by others
Introduction
Macular edema (ME) is characterized by a retinal thickening in the macular area due to the breakdown of the blood-retinal barrier (BRB). Extracellular fluid accumulates in the intraretinal area or collects in the subretinal space. Inflammatory ME may complicate anterior, intermediate or posterior uveitis that may be due to various infectious, neoplastic, or autoimmune etiologies. Uveitis is the fifth leading cause of visual impairment in developed countries and responsible for about 20% of legal blindness.1, 2 ME is the main condition associated with vision loss in uveitis, decreasing the visual acuity (VA) to <20/40 in about one-third of posterior uveitis patients.3, 4 Panuveitis and intermediate uveitis usually occur in conjunction with ME, with an incidence of 66% and 65%, respectively.4
The most common known causes of Uveitis Macular Edema (UME) are HLA B27 positive anterior uveitis, juvenile idiopathic arthritis, intermediate uveitis due to sarcoidosis, multiple sclerosis, and pars-planitis, infections, posterior uveitis due to systemic diseases such as sarcoidosis, Behcet's disease, or due to intra-ocular dysimmunity such as Irvine Gass syndrome post-cataract surgery, Birdshot retinochoroidopathy (retinal vasculitis and depigmented choroiditis associated with HLA-A29), sympathic ophthalmia, and infectious retinitis.5
ME is a significant risk factor for visual loss in uveitis: it has indeed been shown that 45% of patients with posterior uveitis presented with a decrease in VA, and 28% of them also had a ME.4
Therefore in posterior uveitis, ME is the most common complication and the main cause of decreased VA. For example in Birdshot retinochoriopathy, a 5-year cumulative incidence of cystoid ME (CME) of 50% has been shown in eyes free of CME at baseline.6
When uveitis and ME are associated, the visual prognosis depends on the status of the outer retinal layers, and uveitis duration, type, and etiology. A low VA (<20/60) has been found respectively in 64% and 28% of cases of panuveitis and intermediate uveitis alone, and respectively 59% and 85% of them also had a ME.4
UME secondary to anterior and intermediate uveitis can benefit from an early management including work-up and efficient treatment in secondary care center. Chronic, bilateral UME associated with posterior uveitis usually needs a step-wise approach with immunosuppressive therapy, which may be best managed easily in a tertiary care center.
Pathophysiology of UME
The main cause of macular thickening in inflammatory conditions is inflammatory ME. However, other causes can increase the macular thickness in ocular inflammation condition, such as (1) inflammatory choroidal vascularization, (2) vitreo-macular traction by inflammatory epiretinal membrane, (3) contiguity with papillary edema, (4) central serous chorioretinopathy exacerbated by the use of steroid therapy.
Inflammatory ME is due to breakdown of the BRB.The BRB is mainly formed of tight junctions between endothelial cells of non-fenestrated capillaries and retinal pigment epithelial (RPE) cells. Tight junction proteins include zonula occludens, occludins, and VE-cadherins.
At the level of retinal capillary endothelium, the inner BRB breakdown may be due to many factors including vascular endothelium growth factor (VEGF), a signal protein produced by cells stimulating vasculogenesis and angiogenesis that is extensively produced by Müller cells. VEGF modulates occludin and VE-cadherin adhesion and expression; its interaction with its receptor induces a cascade of intracellular phosphorylations causing the degradation of tight junction proteins. Occludin and cadherin phosphorylation, induced by pro-inflammatory cytokines and metalloproteinases secreted by leukocytes, promotes leukocyte migration through intact capillary walls into surrounding body tissue. Diapedesis is a chemotactic process corresponding to the migration of leukocyte toward the retinal tissue and involving their adhesion to the activated endothelium, through the expression of various adhesion proteins, including selectins and ICAM-1 and a conformational change in integrin.7, 8
Factors other than VEGF promoting the BRB breakdown include pro-inflammatory cytokines such as TNF-α, IL-1, TGF-β, angiotensin II, as well as adenosine, histamine, and glucose.7, 8 Increased levels of IL-6 and IL-8 have been found in the aqueous humor of patients with intermediate uveitis.9
At the level of RPE, the outer layer of the BRB, the adhesion between photoreceptors and the RPE is maintained through active transport mechanisms, mainly from the trans-epithelial space to the extraretinal space. That could be damaged in acute inflammatory conditions of the choriocapillaris,10, 11, 12, 13 diffuse choroiditis, and scleritis. Therefore, ME could occur in the presence of healthy retinal capillary endothelium. For exemple, UME usually occurs in VKH menigo-uveitis showing diffuse significant thickened choroiditis, inducing choroidal folds and delayed arterial, capillary, and venous choroidal perfusion, while the retinal vessels used to remain intact as shown using retinal angiography.13
The BRB integrity also depends on other retinal cell types. On one hand, Müller cells, the main type of retinal glial cells, establish connections between the different retinal cell layers and have multiple functions including maintaining the homeostasis of the retinal extracellular milieu through aquaporins and transmembrane potassium channels (Kir). The latter are located on cell interdigitations in the inner retina around retinal capillaries that allow water and potassium transfer to the retinal capillaries. Changes in potassium transport lead to neuronal hyperexcitability and edema. Müller cells also have a neuroprotective role through the release of neurotrophic factors, glutamate uptake and degradation, synthesis of the antioxidant glutathione. They release immunomodulatory cytokines including interleukins (ILs)-1, -13, -4, and -10 which are involved in retinal homeostasis. However, activated Müller cells may also synthesize pro-inflammatory cytokines. Moreover, Müller cell dysfunction impairs glutamate catabolism and dysregulate ion homeostasis, contributing to the development of a retinal edema and neuronal death.
On the other hand, the inner and outer plexiform layers of the retina contain numerous synaptic vesicles and are composed of bipolar, ganglion, and amacrine cells that are involved in the BRB function.
Imaging in UME
Diagnosis is usually made based on imaging examination. Functional signs usually include a drop in near VA; a macular syndrome may be associated with a type of micropsia, metamorphopsia, and positive relative scotoma or blurred vision like looking through a water drop. The visual loss may be highly variable, but in cases with chronic degeneration of the outer retina, the visual effects may be significant. When an acute macular ischemia is associated, like in Behcet's disease, the decrease in VA may persist despite appropriate emergency treatment administration.
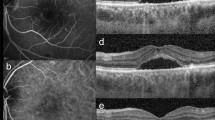
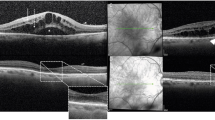
Optical coherence tomography (OCT) is the gold standard technique for the diagnosis of ME, regardless of its etiology, since it is non-invasive, reproducible, and sensitive. It accurately quantifies the retinal macular thickness using mapping, may show fluid accumulation either at the inner (Figures 1 and 2) or outer plexiform layer (Figure 3), a non-uniform photoreceptor outer/inner segment line, the presence of subretinal fluid (Figures 2, 3, 4), hyperreflective dots (Figure 5), epimacular membranes (Figure 6), and vitreomacular traction (Figure 7). Intra-retinal fluid accumulation between retinal septa typically produces a cystoid shape (Figures 1, 2, 3). Other causes of thickened macula in inflammatory conditions are easily detected such as pre-epithelial inflammatory neovascularization (Figure 8) and subretinal fluid in contiguity with papillary edema (Figure 5). OCT also enables analyzing the optic nerve head and the thickness of the optic fibers that could be damaged in uveitic glaucoma.
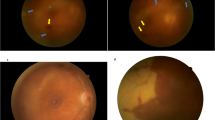
Twenty-year old woman with inflammatory choroidal neovascularization (a): whitish edematous focal deep macular lesion; (b): OCT showing hyper-reflective pre-epithelial focal lesions corresponding to choroidal neovascularization; (c): multiple hyper-reflective dots corresponding to inflammatory intra-retinal deposits.
Retinal fluorescein angiography (FA) usually shows evidence of dye diffusion in the macular area, which may be associated with dye pooling in the cystoid spaces. Recent studies have investigated the concordance between retinal FA and OCT findings for the diagnosis of inflammatory ME, and results have shown a moderate agreement. Among 255 patients (479 eyes), 40% showed angiographic leakage while no ME was evidenced on OCT, and conversely 34% showed ME on OCT without angiographic leakage.14, 15 However, some technical biases may have influenced the results, including time-domain OCT and fast software issues. Apart from inflammatory conditions, discrepancies between OCT and FA findings for ME have been shown by Gaucher et al who have suggested several mechanisms, in particular a very slow protein flow and an intracellular location of the edema.16
In an inflammatory context, retinal FA is still a useful tool. In addition to analyzing the macular ‘leakage’, FA enables assessment of inflammatory retinal lesions. In particular, it allows detecting associated vasculitis, which may be venous, arterial, or mixed, and even sometimes occlusive. It can also provide information about the etiology of uveitis and facilitates the detection of preretinal, prepapillary, or choroidal neovascularization (CNV). Moreover only FA can demonstrate macular ischemia. Finally, it can be used to assess the therapeutic response to the treatment.
Etiological diagnosis
The etiological work-up is guided by initial findings including (1) age, sex, immune status of the patient, (2) intra-ocular and periocular signs, (3) retinal fluorescein and indocyanine green angiography findings, (4) associated systemic features obtained from direct questioning, clinical examination, and ancillary tests.
The age of the patient is an important factor. Before the age of five, main causes include infections (toxoplasmosis, toxocarosis), systemic inflammation such as idiopathic juvenile arthritis and neoplasia (leukaemia, retinoblastoma). In teenagers and young adults, main causes include infections (such as Toxoplasmosis, Herpes virus), systemic inflammation (such as sarcoidosis, spondyloarthropathies, Behcet’s disease, multiple sclerosis, and connective tissue diseases). In the elderly patients the main causes include systemic inflammation (sarcoidosis, BRC), Irvine-Gass syndrome, retinal infections, and neoplasia (lymphoma).
Although compatible with Behcet’s disease and sarcoidosis, unilateral UME often indicates an infectious cause. UME associated with granulomatous corneal precipitates and iris nodules can indicate causes such as sarcoidosis and infectious causes.
UME associated with hypopion can suggest causes such as HLA B27, but may also be compatible with Behcet’s disease that may remain unilateral in the first years, infections, and neoplasia.
UME associated with focal retinitis lesion can also suggest infectious causes, while compatible with Behcet’s disease, that may remain unilateral at the first years of the disease.
The main causes of UME associated with retinal vein vasculitis are summarized (Table 1).
The specific form of ME associated with active retinal phlebitis with candle wax drippings in a relatively broad translucent segmental sleeve is likely to be due to sarcoidosis.
ME may also be secondary to scleritis, choroiditis, or due to acute choriocapillaris lesions inducing outer BRB breakdown. The UME associated with posterior scleritis suggests as main causes: rheumatoid arthritis (RA), Behcet’s disease and SLE, and types of spondyloarthropathy-associated disease, especially Crohn's disease. Scleritis may also be secondary to sarcoidosis or related to herpes virus infection, tuberculosis or spirochetes infection (syphilis, Lyme disease).12 Regarding Vogt–Koyanagi–Harada disease, the ME may be due to polylobed subretinal and intraretinal fluid accumulation associated with vitreous inflammation. In addition, Vogt-Koyanagi–Harada disease-related choroiditis results in major diffuse thickening and secondary choroidal perfusion delay that affects the choroidal arteries, capillaries, and vortices, while no evidence of venous vasculitis is observed in the retina.13
UME associated with retinal arteriolar vasculitis suggests few causes such as infections (Herpes virus, spirochetosis, tuberculosis), systemic vasculitis (Behcet’s disease, connectivitis, and necrotizing angeiitis), idiopathic retinal vasculitis, arteriolar macroaneurysms, and neuroretinitis (IRVAN) syndrome.
UME associated with choroidal nodules (visualised with fluorescein and indocyanine green angiography) suggests causes such as sarcoidosis, BRC, tuberculosis, sympathetic ophthalmia, or numerous micronodules associated with the Vogt–Koyanagi–Harada disease.17, 18, 19 Others deep lesions seen using retinal angiography could be of etiological interest. For example, The choroidal fluorescence blocked by multiple clumps measuring 50 μm in diameter that appear as whitish punctate lesions in the fundus associated with numerous vitreous cells, is suggestive of a non-Hodgkin lymphomatous infiltration.20 Sectorial-shaped choroidal ischemic areas may cause sectorial pigmentary changes in the most severe cases, which may be suggestive of connective tissue disorders such as scleroderma, lupus, polyarteritis nodosa and granulomatosis with polyangiitis, an uncommon inflammation of the blood vessels, resulting in blood flow restriction to major organs.10
The main causes of ME associated with focal retinal and choroidal lesions are summarized in Table 2.
Treatment of inflammatory ME and related AEs
Corticosteroids
In patients with some specific infectious or neoplastic-related ME, the first-line treatment is based on treating the underlying cause. However, ME symptomatic treatment may require the use of anti-inflammatory agents. Corticosteroids are powerful anti-edematous agents and are widely used locally, that is, via subconjunctival, parabulbar, subtenon capsule, or intravitreal injection.
Mild sub-clinical ME could resolve after dexamethasone (DXM) topical treatment alone, especially in acute anterior uveitis, often due to spondyloarthropathies.
Regarding subconjunctival injection, high-DXM levels have been shown in the aqueous humor, and a significant vitreous distribution within the 3 h following its injection.21 Repeated subconjunctival administration may resolve ME complicating severe anterior uveitis (Figure 9). Subtenon triamcinolone injection leads to vitreous concentration similar to those obtained after intravitreal injection, although highly variable vitreous concentrations have been reported, with levels ranging between 0 and 4.94 μg/ml.22 Shen et al have measured the aqueous humor, vitreous, and plasma concentrations after 1subtenon triamcinolone injection and shown that the concentration was twice as high in the vitreous as in the aqueous humor, and 98 times higher than in the plasma.23 Both subconjunctival (10 mg) and parabulbar (20-40 mg) triamcinolone injections result in similar intraocular pressure (IOP) in secondary ocular hypertension with equivalent efficacy found for both routes.24 Intravitreal triamcinolone has been used as an unlicenced preparation. Ozurdex, a slow-release DXM implant, is now available as a licenced preparation for local treatment of non-infectious posterior uveitis. Other longer acting slow release agents are available but are not yet licenced for this indication.
The short-term efficacy of steroid injections is well established. For example in patients with uveitis and ME, a single subtenon injection of triamcinolone has been shown to induce ME resolution in 57% of cases.25 The efficacy is greatest at 1 month after injection, but relapse will normally occur by 6 months following treatment.25 Using a slow-release DXM implant (Ozurdex), a complete resolution of the vitreous inflammation has been observed after 6 months in 47% of 77 uveitis patients with an initial vitreous Tyndall effect of at least 1.5+.26 A significant improvement in persistent inflammatory ME has been shown in terms of centrofoveal thick ness27 and VA in more than 85% of patients at 4 months.28 The efficacy duration may differ and a median time to recurrence is 5 months.29, 30
The fluocinolone acetonide implant (0.2 microg/day Iluvien) releases the drug through 36 months while 0.5 microg/day Retisert releases the drug through 30 months, release timing consistent with the clinical results timing on diabetic ME.28
Regarding adverse events (AEs), corticosteroid-induced glaucoma is well-known. In addition, triamcinolone use can result in secondary hypertension requiring medical treatment after 2 months as shown in 35-65% of patients, and an IOP >21 mm Hg has recently been found in 40.8% of eyes in patients with uveitis associated with Behcet’s disease treated with 4 mg of intravitreal triamcinolone.31 A meta-analysis has shown that 32% of patients had ocular hypertension after having received 4 mg of intravitreal triamcinolone versus 15% after injection of 0.7 mg slow-release DXM implant (Ozurdex).32 Some known predisposing factors include glaucoma, high baseline IOP, young age, ocular hypertension associated with a previous corticotherapy, and the presence of uveitis.25, 32 Because of the high rate of ocular hypertension, glaucoma and cataract associated with intravitreal triamcinolone, its use is now rare, whereas use of Ozurdex is becoming more widespread. Available formulations of Triamcinolone are indicated in the United States for ocular inflammation unresponsive to topical steroids, while in the European Union they remain not approved for any indication.
Lowder et al26 (HURON Study Group) have conducted a prospective, randomized, controlled study to assess the safety and efficacy of 2 doses of DXM intravitreal implant in 229 patients with non-infectious intermediate or posterior uveitis. Seventy-seven patients were injected with 0.7 mg DXM implant (Ozurdex) and 23% of patients required treatment for high IOP at 6 months, while cataracts were found in 15% of phakic eyes at 6 months. More recent studies have found a higher rate of ocular hypertension, for example, an IOP >25 mm Hg has been found in 30% of injected eyes with a peak in ocular hypertension at 2 months.30, 32 However, all these cases of hypertonia have resolved with local treatment. Ozurdex is indicated in the United States, the European Union, and other countries worldwide for the treatment of inflammation of the posterior segment of the eye presenting as non-infectious uveitis. In patients who had received a fluocinolone acetonide implant (Retisert), filtrating surgery was required in 40% of implanted eyes after upto 3 years. Cataracts have been reported in all treated eyes.33 In the United States Retisert is indicated for the treatment of chronic non-infectious uveitis affecting the posterior segment of the eye.
The limitations of topical corticosteroids therefore include glaucoma and cataracts, and they are short-acting molecules since their efficacy is limited to a few months, a period after which retreatment is necessary in cases of recurrence of intraocular inflammation. Furthermore, their use may also delay or exacerbate retinal infection, including herpes virus or toxoplasmosis. Moreover, the intermittent treatment of inflammatory exacerbations has not been shown to prevent the progressive visual loss.34, 35 However, these topical treatments are mainly used in cases of unilateral uveitis, or when the use of systemic corticosteroids is contraindicated, and they can also be used occasionally in association with a systemic treatment (Figure 9). In cases of sight-threatening chronic bilateral posterior uveitis, the systemic route is often necessary and steroids are the standard first-line treatment. Their AEs are well known and include Cushing syndrome, induced diabetes, dyslipidemia, and fluid retention that can be managed in strict compliance with preventive lifestyle changes. When daily glucocorticoid doses exceed 0.2 mg/kg/day to prevent uveitis recurrence, immunosuppressive or immunomodulatory agents are needed for their anti-inflammatory activity and because they are steroid-sparing. Many of the second and third line immunosuppressive agents are unlicensed for this indication in the UK.
Immunosuppressive agents
Conventional immunosuppressive drug classes include calcineurin inhibitors (cyclosporine, tacrolimus), antimetabolites (azathioprine, methotrexate, mycophenolate mofetil MMF) and alkylating agents (cyclophosphamide, chlorambucil). Treatments with azathioprine, cyclosporine A, methotrexate (MTX) or MMF have shown a 83% reduction in ME development during the follow-up of BRC patients.6 Recently, MMF has shown a moderate long-term efficacy with a remission rate of 50% but a ME has appeared in 37% of treated eyes.36 Moreover inhibiting inflammation could benefit patients with chronic inflammatory diseases, such as RA, psoriasis, inflammatory bowel diseases, and systemic lupus erythematosus that had been shown associated with an increased risk of cardiovascular disease.37
AEs of conventional immunosuppressants include a relative risk of malignancy increased by 1.5 (mainly lymphoma, leukaemia, vesical neoplasia), increased risk of infection, medullar, adrenal, and gonadic insufficiency.38 Moreover, each molecule has its own tissue toxicity. Regarding cyclosporine, a renal toxicity has been shown in a prospective study conducted in 27 BRC patients with initially normal renal function treated with cyclosporine A at a dose of 5 mg/kg/d. A 30% drop in glomerular filtration has been shown. After cyclosporine A cessation, an irreversible loss in glomerular filtration has been correlated with the cyclosporine A exposure.39 Moreover chronic kidney disease is thought to be a potential risk factor for cardiovascular death.40
Immunomodulatory agents
Because of these potentially serious AEs, the use of immunomodulatory drugs has been preferred.
Polyvalent immunoglobulins
Polyvalent immunoglobulins (PI) have shown a moderate efficacy on VA which increased by 2 lines in 50% of eyes with an initial VA less than or equal to 20/30. When present, the ME was improved on FA in half of cases.41 Several parameters have limited the wide use of PI, including their human origin which limits their safety to the current knowledge, and their high cost and modest activity on ME. However, only benign AEs such as headache, eczematous lesions, and hyperthermia have been reported.
Anti-TNF and anti-TNFR antibodies
Monoclonal antibodies are used for the treatment of refractory uveitis. The most frequently used are the monoclonal anti-TNF antibodies, including infliximab and adalimumab, whereas the soluble TNF receptor known as etanercept has been shown to be less effective42 and seems related to the occurrence of uveitis.43 Infliximab has shown a rapid efficacy, especially in Behcet’s uveitis, its improvement being observed within a few days.44 A review of published data on the use of anti-TNF agents in Behcet’s disease has shown a clinical ocular response in 89% of 174 patients from 16 prospective open-labeled studies with a median follow-up of 16.2 months. The combination of infliximab with azathioprine and/or cyclosporine A appears superior to infliximab in monotherapy for sustained ocular remission.45 Apart from uveitis in Behcet’s disease, anti-TNF agents, in particular infliximab, may be effective in the treatment of ocular inflammation associated with RA, juvenile idiopathic arthritis, spondyloarthropathy, Crohn’s disease, sarcoidosis, and BRC.46
In refractory uveitis, adalimumab has shown an effect in 68% of 31 patients who responded at 10 weeks, and the response persisted after 50 weeks in 12 of these patients (39%). Thus, 1/3 of patients were unresponsive to initial therapy and further 2/3 became resistant to treatment.47 Its action may be limited to the duration of treatment.48, 49
The efficacy of Golimumab, a novel fully human anti-TNF-α monoclonal antibody, has been shown in uveitis associated with Behcet’s disease, idiopathic juvenile arthritis, and idiopathic retinal vasculitis in very few retrospective case reports.50
One of the potentially serious AEs related to anti-TNF-α antibodies is a reactivation of latent Mycobacterium tuberculosis. Moreover, a wide variety of systemic and organ-specific autoimmune processes, including lupus and lymphoma, have been reported since the early 2000 s. In particular, an aggressive and rare hepatosplenic T-cell lymphoma has been reported in young patients treated for inflammatory bowel disease (Crohn’s disease) with a combination of corticosteroids, thiopurine, and infliximab.51 However, the different sources of pharmacovigilance have failed to address the question of a causal or artifactual association.52 An increased risk of non-melanoma skin cancer has been found associated with the use of a combination of anti-TNF and MTX in RA and with the use of PUVA, cyclosporine, and anti-TNF in psoriasis while the risk of melanoma is slightly increased.53, 54 Thus, biotherapies could be classified according to their potential oncogenic AEs.
Other newer biotherapies
More recently, the efficacy of other monoclonal antibodies, including anti-ILs-1, -2, -6 and -17, has been suggested in refractory uveitis.55 In particular, Daclizumab, an anti-IL-2 antibody, has been used in BRC patients refractory to traditional immunomodulatory therapies. Eight patients reviewed retrospectively have been initially treated with 1 mg/kg of intravenous Daclizumab at 2-week intervals. Over a mean follow-up of 25 months, 7 patients have achieved either a stabilization or an improvement in VA in both eyes and a complete resolution of the vitreous inflammation, while 6 patients have achieved a resolution of the vasculitis on retinal FA. However, 2/8 patients have developed AEs, including increased transaminase levels and leukopenia, leading to daclizumab discontinuation.56
The efficacy of tocilizumab, an anti-IL-6 receptor antibody, has been shown in a few cases of uveitis refractory to anti-TNF-α agents.57, 58
Regarding rituximab, an anti-CD20 monoclonal antibody, it has shown its efficacy in a few cases of various inflammatory eye diseases (uveitis, scleritis, idiopathic inflammatory pseudotumor, or granulomatosis associated with polyangiitis).59
Secukinumab is a human anti-IL-17 monoclonal antibody approved for the treatment of uveitis, RA, and psoriasis. Three multicenter randomized double-blind placebo-controlled dose-ranging phase III studies conducted in 118 patients with uveitis in Behcet’s disease, 125 with quiescent non-infectious non-Behcet’s uveitis and 31 active non-infectious non-Behcet’s uveitis have shown no significant difference in uveitis recurrence between patients treated with secukinumab and the placebo group. However, secukinumab has been associated with a significant reduction in mean number of total post-baseline immunosuppressive medications used in one of the study (SHIELD study). No visual loss has been reported in any treatment group during the follow-up in the three studies.60
Interferon (IFN) therapies
Other immunomodulatory drugs are interferons (IFNs), which are intracellular cytokines. IFN-α mediates a wide range of biological effects, including direct antiviral, immunomodulatory, antiproliferative, antiangiogenic and antitumor activities. IFN-α can upregulate the class I and class II major histocompatibility complex, induce natural killer cell activity, and enhance dendritic cell activation.61
The efficacy of IFN-α and IFN-β has been shown in the treatment of inflammatory ME. A meta-analysis of patients with Behcet’s disease has shown an ocular response in 94% of patients with a 50% decrease in disease activity after 4 weeks.62 The efficacy of IFN-α has also been shown in more than 75% of Behcet’s uveitis patients.63, 64, 65 A sixfold decrease in mean uveitis attack rate has been observed on treatment compared with previous treatment phases.64, 65, 66
The efficacy of IFNs has been suggested in non-Behcet ME such as BRC67 and idiopathic uveitis.68, 69 For example, among 12 patients treated with daily IFN-α, 83% presented a response and 100% of ME resolved.70
A single-centre prospective, randomized study has compared the efficacy of 44 mcg of subcutaneous IFN-β 3 times per week versus 20 mg of subcutaneous MTX once a week in the treatment of ME complicated by uveitis. The low dose of IFN-β appeared more effective than MTX, with respectively a mean decrease in macular thickness of 206 μm versus a mean increase of 47 μm.71 However, it should be noted that MTX is not considered a standard treatment for inflammatory ME.
One of the most limiting effects of IFN treatment is the occurrence of numerous AEs. Its immediate AEs, including fever, arthralgia, and flu-like syndrome, are usually controlled with oral paracetamol. IFN-α-induced depression is a common and dangerous AE.72 For example, among 11 patients with posterior uveitis treated daily with 6 million international units (MIU) of IFN-α2b for at least 2 weeks, 4 experienced depression, and one of them attempted suicide.70 Psychiatric side effects has tend to limit INF use to a greater extent. Other common AEs of IFN-α include leukopenia, thrombocytopenia, and occurrence of serum antinuclear, anti-DNA, antithyroid antibodies. Overt clinical autoimmune thyroid disease, autoimmune hepatitis, and sarcoidosis lesions with positive skin biopsy have also been reported.73
In addition, inflammatory and vascular intraocular AEs have been reported with IFN-α and may limit its use in posterior uveitis. A few case reports of Vogt–Koyanagi–Harada-like disease, a T-cell-mediated autoimmune response against melanocytes, have been described.74, 75, 76 Furthermore, a few case reports of granulomatous panuveitis suggesting sarcoidosis have also been described in HCV-infected patients treated with ribavirin in combination with IFN-α2b, pegylated or not. Only 1 patient required systemic corticosteroids.77
In 29-60% of IFN-α-treated hepatitis patients, IFN-associated retinopathy including retinal arteriolar or venular occlusion78 usually occurs within the first 6 months of treatment and resolves while treatment is continued.79
The conventional treatment of ME in Irvin-Gass syndrome includes acetazolamide, a carbonic anhydrase inhibitor that enhances retinal liquid transport to the choroid, whose AEs include paresthesia, hypokalemia, sulfur allergy, nausea, stomach pain, and renal colic, needing preventive urinary alkalinization and potassium supplementation.
Benefits of intravitreal injections
Intravitreal injections of molecules other than corticosteroids have shown an experimental and clinical interest. Intravitreal IFN-α2b has been tested in rabbit eyes and it has shown a good tolerance for doses up to 1 MIU.80 A single intravitreal injection of infliximab (1 mg) has been shown to decrease inflammation in the aqueous humor, vitreous, and retinal veins although a persistent ME was observed in 9/11 eyes.81 Intravitreal injections of bevacizumab, an anti-VEGF monoclonal antibody widely used in the treatment of CNV in age-related macular degeneration and diabetic ME, have shown a transient positive effect on macular thickness.82 Another retrospective study has compared 3 groups treated with either 1.25 mg of intravitreal bevacizumab, 4 mg of intravitreal triamcinolone acetonide, or 40 mg of posterior subtenon triamcinolone acetonide. The respective mean central foveal thickness decreased from baseline by 167, 327, 166 μm. Kaplan–Meier survival analysis has shown that the median effect duration was respectively 16, 30, and 12 weeks. A 5- mmHg increase in IOP was observed in 10, 45, and 40% of eyes, respectively.83 However, in another randomized study comparing intravitreal bevacizumab and triamcinolone injections, the bevacizumab-treated group did not show any significant decrease in central foveal thickness.84 Moreover, acute intraocular inflammation has been described with an incidence of 1.30% in eyes having received a total of 693 intravitreal bevacizumab injections.85
The efficacy of intravitreal MTX injections (400 μg) has been shown on the control of the intraocular inflammation with an increase in VA in 30/38 (79%) injected eyes. Among the responsive eyes, 73% achieved a sustained response with a mean time to relapse of 17 months.86 Side effects were described while MTX intravitreal injections were used for treating vitreoretinal lymphoma. The most common cause was corneal epitheliopathy, which usually appeared after the third injection and began to subside when the intervals between injections increased.87
The efficacy of sirolimus has been suggested on vitreous inflammation without significant difference between subconjonctival and intravitreal injections.88
In case of ME related to inflammatory CNV, the long-term efficacy of intravitreal bevacizumab has been reported by Mansour et al in a study of 81 patients with uveitis.89 They have observed a significant increase in VA in patients with histoplasmosis, multifocal choroiditis, punctate inner chorioretinopthy and toxoplasmosis, as well as a significant decrease in foveal thickness in the overall patient group. At 3 years, significant sustained visual improvement of 2.7 lines and foveal flattening of 98 μm have been observed in numerous inflammatory ocular diseases without major complications after a median of 3 intravitreal bevacizumab injections.89 Prospective studies are ongoing to assess the efficacy of intravitreal ranibizumab and aflibercept on inflammatory CNV.
Preventive treatment
Surgical treatment of vitreoretinal traction and epiretinal membrane is rarely beneficial, but if performed, it should be associated with an effective immunosuppressive treatment because of the high risk of increased intraocular inflammation during the immediate post-surgery period. Anti-inflammatory eye drop formulations either alone or in combination with topical prednisolone may be more effective to prevent the onset of Irvine-Gass syndrome than prednisolone alone.90 The recurrence of ME during the period following cataract surgery may be prevented in eyes with history of uveitis using an effective immunosuppressive treatment at the time of the surgery and with a closer follow-up for prompt treatment in case of post-operative inflammation recurrence.
Conclusion
In conclusion, the set of topical intravitreal therapies available is expanding, with the current use of corticosteroids followed by the use of immunosuppressive agents. However, severe bilateral uveitis still requires systemic treatments and it is anticipated that new drugs effective in this indication will continue to emerge, but prospective controlled studies will be needed for their assessment. However, at this stage of drug development, we recommend following the proposed therapeutic algorithm (Figure 9) for the treatment of non-infectious UVE.
References
Gritz DC, Wong IG . Incidence and prevalence of uveitis in Northern California; the Northern California Epidemiology of Uveitis Study. Ophthalmology 2004; 111: 491–500.
Suhler EB, Lloyd MJ, Choi D, Rosenbaum JT, Austin DF . Incidence and prevalence of uveitis in Veterans Affairs Medical Centers of the Pacific Northwest. Am J Ophthalmol 2008; 146: 890–896.
Lardenoye CW, van Kooij B, Rothova A . Impact of macular edema on visual acuity in uveitis. Ophthalmology 2006; 113: 1446–1449.
Rothova A, Suttorp-van Schulten MS, Frits Treffers W, Kijlstra A . Causes and frequency of blindness in patients with intraocular inflammatory disease. Br J Ophthalmol 1996; 80: 332–336.
Bajwa A, Osmanzada D, Osmanzada S, Khan I, Patrie J, Xin W et al. Epidemiology of uveitis in the mid-Atlantic United States. Clin Ophthalmol 2015; 9: 889–901.
Thorne JE, Jabs DA, Peters GB, Hair D, Dunn JP, Kempen JH . Birdshot retinochoroidopathy: ocular complications and visual impairment. Am J Ophthalmol 2005; 140: 45–51.
Omri S, Behar-Cohen F, de Kozak Y, Sennlaub F, Verissimo LM, Jonet L et al. Microglia/macrophages migrate through retinal epithelium barrier by a transcellular route in diabetic retinopathy: role of PKCζ in the Goto Kakizaki rat model. Am J Pathol 2011; 179: 942–953.
Yhuel Y, Weber M . Physiopathologie de l’oedème maculaire inflammatoire. In: Cohen SY, Gaudric A (Eds), Rétine. Médecine Sciences Publications, 2012, pp 184–189.
Valentincic NV, de Groot-Mijnes JD, Kraut A, Korosec P, Hawlina M, Rothova A . Intraocular and serum cytokine profiles in patients with intermediate uveitis. Mol Vis 2011; 17: 2003–2010.
Gaudric A, Privat E . Ischémie choroïdienne aigue. In: Pournaras CJ (eds), Pathologies vasculaires oculaires, Société Française d’Ophtalmologie: Paris, France, 2008, pp 555–571.
Wright BE, Bird AC, Hamilton AM . Placoid pigment epitheliopathy and Harada’s disease. Br J Ophthalmol 1978; 62: 609–621.
Pavesio CE, Meier FM . Systemic disorders associated with episcleritis and scleritis. Curr Opin Ophthalmol 2001; 12: 471–478.
Fardeau C, Tran TH, Gharbi B, Cassoux N, Bodaghi B, LeHoang P . Retinal fluorescein and indocyanine green angiography and optical coherence tomography in successive stages of Vogt-Koyanagi-Harada disease. Int Ophthalmol 2007; 27: 163–172.
Kempen JH, Sugar EA, Jaffe GJ, Acharya NR, Dunn JP, Elner SG et al. Fluorescein angiography versus optical coherence tomography for diagnosis of uveitic macular edema. Ophthalmology 2013; 120: 1852–1859.
Tran TH, de Smet MD, Bodaghi B, Fardeau C, Cassoux N, Lehoang P . Uveitic ME: correlation between optical coherence tomography patterns with visual acuity and fluorescein angiography. Br J Ophthalmol 2008; 92: 922–927.
Gaucher D, Saleh M, Sauer A, Speeg-Schatz C, Bourcier T, Gaudric A . [Macular edema without fluorescein leakeage]. J Fr Ophtalmol 2009; 32: 314–319.
Fardeau C, Herbort CP, Kullmann N, Quentel G, LeHoang P . Indocyanine green angiography in birdshot chorioretinopathy. Ophthalmology 1999; 106: 1928–1934.
Herbort CP, LeHoang P, Guex-Crosier Y . Schematic interpretation of indocyanine green angiography in posterior uveitis using a standard protocol. Ophthalmology 1998; 105: 432–440.
Papadia M, Herbort CP, Mochizuki M . Diagnosis of ocular sarcoidosis. Ocul Immunol Inflamm 2010; 18: 432–441.
Fardeau C, Lee C.P.L, Merle-Béral H, Cassoux N, Bodaghi B, Davi F et al. Retinal fluorescein, indocyanine green angiography, and optic coherence tomography in non-Hodgkin’s primary intraocular lymphoma. Am J Ophthalmol 2009; 147: 886–894.
Weijtens O, Feron EJ, Schoemaker RC, Cohen AF, Lentjes EG, Romijn FP et al. High concentration of dexamethasone in aqueous and vitreous after subconjonctival injection. Am J Ophthalmol 1999; 128: 192–197.
Thomas ER, Wang J, Ege E, Madsen R, Hainswort DP . Intravitreal triamcinolone acetonide concentration after subtenon injection. Am J Ophthalmol 2006; 142: 860–861.
Shen L, You Y, Sun S, Chen Y, Qu J, Cheng L . Intraocular and systemic pharmacokineticsof triamcinolone acetonideafter a single 40mg posterior subtenon application. Ophthalmology 2010; 117: 2365–2371.
Blériot A, Couret C, Le Meur G, Lebranchu P, Weber M . [Safety and efficacy of subconjuctival triamcinolone injections in the management of uveitis macular edema]. J Fr Ophtalmol 2014; 37: 599–604.
Leder HA, Jabs DA, Galor A, Dunn JP, Thorne JE . Periocular triamcinolone acetonide injections for cystoid macular edema complicating noninfectious uveitis. Am J Ophthalmol 2011; 152: 441–448.
Lowder C, Belfort Jr R, Lightman S, Foster CS, Robinson MR, Schiffman RM et alOzurdex HURON Study Group. Dexamethasone intravitreal implant for noninfectious intermediate or posterior uveitis. Arch Ophthalmol 2011; 129: 545–553.
Adán A, Pelegrín L, Rey A, Llorenç V, Mesquida M, Molins B et al. Dexamethasone intravitreal implant for treatment of uveitic persistent cystoid macular edema in vitrectomized patients. Retina 2013; 33: 1435–1440.
Campochiaro PA, Brown DM, Pearson A, Ciulla T, Boyer D, Holz FG et al. Long-term benefit of sustained-delivery fluocinolone acetonide vitreous inserts for diabetic macular edema. Ophthalmology 2011; 118: 626–635.
Habot-Wilner Z, Sallam A, Pacheco PA, Do HH, McCluskey P, Lightman S . Intravitreal triamcinolone acetonide as adjunctive treatment with systemic therapy for uveitic macular edema. Eur J Ophthalmol 2011; 21 (Suppl 6): S56–S61.
Champion E, Cardoso J, Darugar A, Fel A, Touitou V, LeHoang P et al. Intravitreal dexamethasone implant in non-infectious uveitis: a one-year follow-up. Poster section Uveitis, ARVO, 2014.
Park UC, Park JH, Yu HG . Long-term outcome of intravitreal triamcinolone acetonide injection for the treatment of uveitis attacks in Behçet disease. Ocul Immunol Inflamm 2014; 22: 27–33.
Kiddee W, Trope GE, Sheng L, Beltran-Agullo L, Smith M, Strungaru MH et al. Intraocular pressure monitoring post intravitreal steroids: a systematic review. Surv Ophthalmol 2013; 58 (4): 291–310.
Pavesio C, Zierhut M, Bairi K, Comstock TL, Usner DW . Fluocinolone Acetonide Study Group. Evaluation of an intravitreal fluocinolone acetonide implant versus standard systemic therapy in noninfectious posterior uveitis. Ophthalmology 2010; 117: 567–575.
Oh KT, Christmas NJ, Folk JC . Birdshot retinochoroiditis: long term follow-up of a chronically progressive disease. Am J Ophthalmol 2002; 133: 622–629.
Rothova A, Berendschot TT, Probst K, van Kooij B, Baarsma GS . Birdshot chorioretinopathy: long-term manifestations and visual prognosis. Ophthalmology 2004; 111: 954–959.
Doycheva D1, Zierhut M, Blumenstock G, Stuebiger N, Deuter C . Mycophenolate mofetil in the therapy of uveitic macular edema—long-term results. Ocul Immunol Inflamm 2012; 20: 203–211.
Lindhardsen J1, Kristensen SL, Ahlehoff O . Management of Cardiovascular Risk in Patients with Chronic Inflammatory Diseases: Current Evidence and Future Perspectives. Am J Cardiovasc Drugs 2016; 16: 1–8.
Kuijken I1, Bavinck JN . Skin cancer risk associated with immunosuppressive therapy in organ transplant recipients: epidemiology and proposed mechanisms. BioDrugs 2000; 14: 319–329.
Tostivint I, du Montcel ST, Jaudon MC, Mallet A, Le Hoang P, Bodaghi B et al. Renal outcome after ciclosporin-induced nephrotoxicity. Nephrol Dial Transplant 2007; 22: 880–885.
Poulikakos D, Banerjee D, Malik M . Risk of sudden cardiac death in chronic kidney disease. J Cardiovasc Electrophysiol 2014; 25: 222–231.
LeHoang P, Cassoux N, George F, Kullmann N, Kazatchkine MD . Intravenous immunoglobulin (IVIg) for the treatment of birdshot retinochoroidopathy. Ocul Immunol Inflamm 2000; 8: 49–57.
Galor A, Perez VL, Hammel JP, Lowder CY . Differential effectiveness of etanercept and infliximab in the treatment of ocular inflammation. Ophthalmology 2006; 113: 2317–2323.
Tynjälä P, Lindahl P, Honkanen V, Lahdenne P, Kotaniemi K . Infliximab and etanercept in the treatment of chronic uveitis associated with refractory juvenile idiopathic arthritis. Ann Rheum Dis 2007; 66: 548–550.
Sfikakis PP, Theodossiadis PG, Katsiari CG, Kaklamanis P, Markomichelakis NN . Effect of infliximab on sight-threatening panuveitis in Behçet's disease. Lancet 2001; 358: 295–296.
Arida A, Fragiadaki K, Giavri E, Sfikakis PP . Anti-TNF agents for Behçet's disease: analysis of published data on 369 patients. Semin Arthritis Rheum 2011; 41: 61–70.
Theodossiadis PG, Markomichelakis NN, Sfikakis PP . Tumor necrosis factor antagonists: preliminary evidence for an emerging approach in the treatment of ocular inflammation. Retina 2007; 27: 399–413.
Suhler EB, Lowder CY, Goldstein DA, Giles T, Lauer AK, Kurz PA et al. Adalimumab therapy for refractory uveitis: results of a multicentre, open-label, prospective trial. Br J Ophthalmol 2013; 97: 481–486.
Sfikakis PP . The first decade of biologic TNF antagonists in clinical practice: lessons learned, unresolved issues and future directions. Curr Dir Autoimmun 2010; 11: 180–210.
Sharma SM, Nestel AR, Lee RW, Dick AD . Clinical review: Anti-TNFalpha therapies in uveitis: perspective on 5 years of clinical experience. Ocul Immunol Inflamm 2009; 17: 403–414.
Mesquida M, Victoria Hernández M, Llorenç V, Pelegrín L, Espinosa G, Dick AD et al. Behçet disease-associated uveitis successfully treated with golimumab. Ocul Immunol Inflamm 2013; 21: 160–162.
Herrinton LJ, Liu L, Chen L, Harrold LR, Raebel MA, Curtis JR et al. Association between anti-TNF-α therapy and all-cause mortality. Pharmacoepidemiol Drug Saf 2012; 21: 1311–1320.
Beigel F, Steinborn A, Schnitzler F, Tillack C, Breiteneicher S, John JM et al. Risk of malignancies in patients with inflammatory bowel disease treated with thiopurines or anti-TNF alpha antibodies. Pharmacoepidemiol Drug Saf 2014; 23: 735–744.
Beyaert R, Beaugerie L, Van Assche G, Brochez L, Renauld JC, Viguier M et al. Cancer risk in immune-mediated inflammatory diseases (IMID). Mol Cancer 2013; 12: 98.
Ramiro S, Gaujoux-Viala C, Nam JL, Smolen JS, Buch M, Gossec L et al. Safety of synthetic and biological DMARDs: a systematic literature review informing the 2013 update of the EULAR recommendations for management of rheumatoid arthritis. Ann Rheum Dis 2014; 73: 529–535.
Imrie FR, Dick AD . Nonsteroidal drugs for the treatment of noninfectious posterior and intermediate uveitis. Curr Opin Ophthalmol 2007; 18: 212–219.
Sobrin L, Huang JJ, Christen W, Kafkala C, Choopong P, Foster CS . Daclizumab for treatment of birdshot chorioretinopathy. Arch Ophthalmol 2008; 126: 186–191.
Muselier A, Bielefeld P, Bidot S, Vinit J, Besancenot JF, Bron A . Efficacy of tocilizumab in two patients with anti-TNF-alpha refractory uveitis. Ocul Immunol Inflamm 2011; 19: 382–383.
Adán A, Mesquida M, Llorenç V, Espinosa G, Molins B, Hernández MV et al. Tocilizumab treatment for refractory uveitis-related cystoid macular edema. Graefes Arch Clin Exp Ophthalmol 2013; 251: 2627–2632.
Miserocchi E, Modorati G . Rituximab for noninfectious uveitis. Dev Ophthalmol 2012; 51: 98–109.
Dick AD, Tugal-Tutkun I, Foster S, Zierhut M, Melissa Liew SH, Bezlyak V et al. Secukinumab in the treatment of noninfectious uveitis: results of three randomized, controlled clinical trials. Ophthalmology 2013; 120: 777–787.
Theofilopoulos AN, Baccala R, Beutler B, Kono DH . Type I interferons (alpha/beta) in immunity and autoimmunity. Annu Rev Immunol 2005; 23: 307–336.
Kötter I, Günaydin I, Zierhut M, Stübiger N . The use of INFalpha in Behçet disease: review of the literature. Review. Semin Arthritis Rheum 2004; 33: 320–335.
Georgiou S, Monastirli A, Pasmatzi E, Gartaganis S, Goerz G, Tsambaos D . Efficacy and safety of systemic recombinant interferon-alpha in Behçet’s disease. J Intern Med 1998; 243: 367–372.
Gueudry J, Wechsler B, Terrada C, Gendron G, Cassoux N, Fardeau C et al. Long-term efficacy and safety of low-dose interferon alpha2a therapy in severe uveitis associated with Behçet disease. Am J Ophthalmol 2008; 146: 837–844.
Sobaci G, Erdem U, Durukan AH, Erdurman C, Bayer A, Köksal S et al. Safety and effectiveness of interferon alpha-2a in treatment of patients with Behçet’s uveitis refractory to conventional treatments. Ophthalmology 2010; 117: 1430–1435.
Onal S, Kazokoglu H, Koc A, Akman M, Bavbek T, Direskeneli H et al. Long-term efficacy and safety of low-dose and dose-escalating interferon alfa-2a therapy in refractory Behçet uveitis. Arch Ophthalmol 2011; 129: 288–294.
Fardeau C . Interferon and retinal vasculitis. J Fr Ophtalmol 2006; 29: 392–397.
Bodaghi B, Gendron G, Wechsler B, Terrada C, Cassoux N, Huong du LT et al. Efficacy of interferon alpha in the treatment of refractory and sight threatening uveitis: a retrospective monocentric study of 45 patients. Br J Ophthalmol 2007; 91: 335–339.
Deuter CM, Kötter I, Günaydin I, Stübiger N, Doycheva DG, Zierhut M . Efficacy and tolerability of interferon alpha treatment in patients with chronic cystoid macular oedema due to non-infectious uveitis. Br J Ophthalmol 2009; 93: 906–913.
Plskova J, Greiner K, Forrester JV . Interferon-alpha as an effective treatment for noninfectious posterior uveitis and panuveitis. Am J Ophthalmol 2007; 144: 55–61.
Mackensen F, Jakob E, Springer C, Dobner BC, Wiehler U, Weimer P et al. Interferon versus methotrexate in intermediate uveitis with macular edema: results of a randomized controlled clinical trial. Am J Ophthalmol 2013; 156: 478–486.
Reichenberg A, Gorman JM, Dieterich DT . Interferon-induced depression and cognitive impairment in hepatitis C virus patients: a 72 week prospective study. AIDS 2005; 19: S174–S178.
Vispo E, Maida I, Moreno A, Barreiro P, Soriano V . Autoimmune hepatitis induced by pegylated interferon in an HIV-infected patient with chronic hepatitis C. J Antimicrob Chemother 2008; 62: 1470–1472.
Sylvestre DL1, Disston AR, Bui DP . Vogt-Koyanagi-Harada disease associated with interferon alpha-2b/ribavirin combination therapy. J Viral Hepat 2003; 10: 467–470.
Schulman JA, Liang C, Kooragayala LM, King J . Posterior segment complications in patients with hepatitis C treated with interferon and ribavirin. Ophthalmology 2003; 110: 437–442.
Touitou V, Bodaghi B, Cassoux N, Tran TH, Rao NA, Cacoub P et al. Vogt-Koyanagi-Harada disease in patients with chronic hepatitis C. Am J Ophthalmol 2005; 140: 949–952.
Doycheva D, Deuter C, Stuebiger N, Zierhut M . Interferon-alpha-associated presumed ocular sarcoidosis. Graefes Arch Clin Exp Ophthalmol 2009; 247: 675–680.
Tokai R, Ikeda T, Miyaura T, Sato K . Interferon-associated retinopathy and cystoid macular edema. Arch Ophthalmol 2001; 119: 1077–1079.
Cuthbertson FM, Davies M, McKibbin M . Is screening for interferon retinopathy in hepatitis C justified? Br J Ophthalmol 2004; 88: 1518–1520.
Kertes PJ, Britton Jr WA, Addison DJ, Munro SM, Marshall DH, Leonard BC . Toxicity of intravitreal interferon alpha-2b in the rabbit. Can J Ophthalmol 1995; 30: 355–359.
Markomichelakis N, Delicha E, Masselos S, Sfikakis PP . Intravitreal infliximab for sight-threatening relapsing uveitis in Behçet disease: a pilot study in 15 patients. Am J Ophthalmol 2012; 154: 534–541.
Mackensen F, Heinz C, Becker MD, Heiligenhaus A . Intravitreal bevacizumab (avastin) as a treatment for refractory macular edema in patients with uveitis: a pilot study. Retina 2008; 28: 41–45.
Bae JH, Lee CS, Lee SC . Efficacy and safety of intravitreal bevacizumab compared with intravitreal and posterior sub-tenon triamcinolone acetonide for treatment of uveitic cystoid macular edema. Retina 2011; 31: 111–118.
Rabbanikhah Z, Ramezani A, Kiavash V, Yaseri M, Peyman GA . Intravitreal bevacizumab versus triamcinolone acetonide for refractory uveitic cystoid macular edema: a randomized pilot study. J Ocul Pharmacol Ther 2010; 26: 199–206.
Johnson D, Hollands H, Hollands S, Sharma S . Incidence and characteristics of acute intraocular inflammation after intravitreal injection of bevacizumab: a retrospective cohort study. Can J Ophthalmol 2010; 45: 239–242.
Taylor SR, Banker A, Schlaen A, Couto C, Matthe E, Joshi L et al. Intraocular methotrexate can induce extended remission in some patients in noninfectious uveitis. Retina 2013; 33: 2149–2154.
Frenkel S, Hendler K, Siegal T, Shalom E, Pe'er J . Intravitreal methotrexate for treating vitreoretinal lymphoma: 10 years of experience. Br J Ophthalmol 2008; 92: 383–388.
Nguyen QD, Ibrahim MA, Watters A, Bittencourt M, Yohannan J, Sepah YJ et al. Ocular tolerability and efficacy of intravitreal and subconjunctival injections of sirolimus in patients with non-infectious uveitis: primary 6-month results of the SAVE Study. J Ophthalmic Inflamm Infect 2013; 3: 32.
Mansour AM, Arevalo JF, Fardeau C, Hrisomalos EN, Chan WM, Lai TY et al. Three-year visual and anatomic results of administration intravitreal bevacizumab in inflammatory ocular neovascularisation. Can J Ophthalmol 2012; 47: 269–274.
Wolf EJ, Braunstein A, Shih C, Braunstein RE . Incidence of visually significant pseudophakic macular edema after uneventful phacoemulsification in patients treated with nepafenac. J Cataract Refract Surg 2007; 33: 1546–1549.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Rights and permissions
About this article
Cite this article
Fardeau, C., Champion, E., Massamba, N. et al. Uveitic macular edema. Eye 30, 1277–1292 (2016). https://doi.org/10.1038/eye.2016.115
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/eye.2016.115
This article is cited by
-
Cystoid Macular Oedema in a Patient Treated with STING Agonist and Ezabenlimab for Disseminated Melanoma
Ophthalmology and Therapy (2024)
-
Immunity status and expression of molecular markers (ICAM-1, CD5, CD25, CD95) on lymphocytes of patients with recurrent anterior uveitis complicated by macular edema
Graefe's Archive for Clinical and Experimental Ophthalmology (2023)
-
Vitreoretinal surgery in the management of infectious and non-infectious uveitis — a narrative review
Graefe's Archive for Clinical and Experimental Ophthalmology (2023)
-
Comparison of hyperreflective foci in macular edema secondary to multiple etiologies with spectral-domain optical coherence tomography: An observational study
BMC Ophthalmology (2022)
-
Ciclosporin A in bilateral auto-immune chronic posterior uveitis associated with macular oedema: a Long-term Observational Safety and Efficacy Study
Eye (2022)