Abstract
Vascular smooth muscle cells (VSMCs) undergo phenotypic changes in response to vascular injury such as angioplasty. Protein kinase G (PKG) has an important role in the process of VSMC phenotype switching. In this study, we examined whether rosiglitazone, a peroxisome proliferator-activated receptor (PPAR)-γ agonist, could modulate VSMC phenotype through the PKG pathway to reduce neointimal hyperplasia after angioplasty. In vitro experiments showed that rosiglitazone inhibited the phenotype change of VSMCs from a contractile to a synthetic form. The platelet-derived growth factor (PDGF)-induced reduction of PKG level was reversed by rosiglitazone treatment, resulting in increased PKG activity. This increased activity of PKG resulted in phosphorylation of vasodilator-stimulated phosphoprotein at serine 239, leading to inhibited proliferation of VSMCs. Interestingly, rosiglitazone did not change the level of nitric oxide (NO) or cyclic guanosine monophosphate (cGMP), which are upstream of PKG, suggesting that rosiglitazone influences PKG itself. Chromatin immunoprecipitation assays for the PKG promoter showed that the activation of PKG by rosiglitazone was mediated by the increased binding of Sp1 on the promoter region of PKG. In vivo experiments showed that rosiglitazone significantly inhibited neointimal formation after balloon injury. Immunohistochemistry staining for calponin and thrombospondin showed that this effect of rosiglitazone was mediated by modulating VSMC phenotype. Our findings demonstrate that rosiglitazone is a potent modulator of VSMC phenotype, which is regulated by PKG. This activation of PKG by rosiglitazone results in reduced neointimal hyperplasia after angioplasty. These results provide important mechanistic insight into the cardiovascular-protective effect of PPARγ.
Similar content being viewed by others
Introduction
Vascular diseases such as atherosclerosis and hypertension are among the most common causes of morbidity and mortality worldwide. Moreover, the prevalence of those diseases has been rapidly increasing. Therefore, vascular protection has become important in decreasing cardiovascular mortality and morbidity. To treat cardiovascular disease patients, drug therapies have received much attention. For example, thiazolidinediones have pleiotropic effects on the cardiovascular system, in addition to lowering blood glucose.1, 2, 3, 4, 5 Rosiglitazone modulates cardiovascular risk factors through its anti-inflammatory, anti-atherogenic and anti-thrombotic properties.1, 4 Considering vascular pathophysiology, all these anti-atherogenic and anti-restenotic effects appear to be related to modulating the properties of vascular smooth muscle cells (VSMCs). The proliferation and migration of VSMCs have pivotal roles in the progression of atherosclerosis and the development of restenosis after vascular interventions.1
VSMCs can be divided into two types, contractile and synthetic/proliferative.6, 7 The synthetic VSMCs contribute to the progression of atherosclerosis and the formation of neointimal hyperplasia after vascular injury.6 Therefore, it is important to modulate the phenotype change of VSMCs to block the progression of atherosclerosis and restenosis. Among other molecular pathways, protein kinase G (PKG) has a pivotal role in modulating VSMC phenotype.8
Here, we tested whether rosiglitazone could have vascular-protective effects by modulating VSMC phenotype, and if so, whether the activation of PKG underlies rosiglitazone’s effects on VSMCs.
Materials and Methods
Materials
Rosiglitazone and GW9662 were supplied from GlaxoSmithKline (GlaxoSmithKline UK Ltd., Middlesex, UK) and dissolved in dimethylsulfoxide. Recombinant rat PDGF-BB was purchased from R&D systems (Minneapolis, MN, USA) and mithramycin from Sigma-Aldrich (St Louis, MO, USA). Anti-PKG I, anti-PKG Iα, anti-PKG Iβ and goat anti-actin antibodies were obtained from Santa Cruz Biotechnology (Santa Cruz, CA, USA). Rabbit anti-vasodilator-stimulated phosphoprotein (VASP; Ser239) and rabbit anti-total VASP were purchased from Cell Signaling (Berkeley, MA, USA). Mouse anti-calponin antibody was obtained from Sigma-Aldrich. Mouse anti-α-smooth muscle actin (SMA) antibody was purchased from Abcam (Cambridge, MA, USA). An Alzet osmotic pump was purchased from Durect corporation (Cupertino, CA, USA).
Cell isolation and culture
Rat aortic VSMCs were isolated from the thoracic aorta of Sprague–Dawley rats by enzymatic dispersion using a previously described method with minor modification.9 Cells were cultured in Dulbecco’s modified Eagle’s medium supplemented with 10% fetal bovine serum with penicillin/streptomycin in a 37 °C, 5% CO2 incubator.
Cell viability and proliferation assay
Cell viability and proliferation were measured using the trypan blue exclusion assay and the incorporation of bromodeoxyuridine (Roche Molecular Biochemicals, Indianapolis, IN, USA), respectively, according to the manufacturer’s instructions. After serum starvation, rosiglitazone was added and the cells were stimulated with PDGF-BB. At the end of the incubation period, the bromodeoxyuridine was added and the cells were incubated for another 4 h.
Immunofluorescence staining
For immunofluorescence staining, cells were washed twice with PBS and fixed with 100% cold methanol for 10 min in −20 °C. After washing away the methanol with 0.05% TBS-T three times, blocking was performed with a 1% bovine serum albumin solution. Immunofluorescence staining was performed using anti-α-SMA, anti-calponin and anti-thrombospondin antibodies. Nuclei were stained with 4′-6-diamidino-2-phenylindole.
Western blot analysis
VSMCs were stimulated using 10 ng ml−1 of PDGF-BB with or without 10 μM rosiglitazone for the indicated times after serum starvation. Immunoblot assays were performed as described previously.10, 11
Reverse transcription-PCR
Total RNA was extracted from VSMCs using TRIzol reagent (Invitrogen, Carlsbad, CA, USA). One microgram of total RNA was reverse-transcribed using the reverse transcription system. PCR was performed using the following paired primers: forward 5′-TTGGTCTTGTTAGCCTAGTC-3′ and reverse 5′-TGTGCAGTCCCAGTGAGGAAC-3′ for inducible nitric oxide synthase; and forward 5′-CAGAACATCATCCCTGCCTCT-3′ and reverse 5′-GCTTGACAAAGTGGTCGTTGAG-3′ for glyceraldehyde 3-phosphate dehydrogenase.
NO and cGMP measurement
The cell culture supernatant was collected after various times, and NO concentration was determined with the Griess Reagent System (Promega, Madison, WI, USA). cGMP concentration in the medium was measured using a Cyclic GMP Assay kit (R&D systems) according to the manufacturer's instructions.
Chromatin immunoprecipitation (ChIP) assay
To evaluate whether Sp1 binds to the Sp1-binding site of the rat PKG promoter, a ChIP assay was carried out following the ChIP Assay Kit protocol (Upstate Biotechnology, Lake Placid, NY, USA). The cells were treated with rosiglitazone or mithramycin and then stimulated with PDGF-BB. The sonicated lysate was used as an input control, and the remaining lysate was for immunoprecipitation with or without anti-Sp1 antibodies (Santa Cruz Biotechnology). The precipitated DNA fragments were analyzed by PCR with primers for the PKG promoter using the forward primer 5′-GGATCCAGTTACAAGCACT G-3′ and the reverse primer 5′-CTCCTGCTGAATGGACTAGA-3′.
Rat carotid artery balloon injury model
All animal experiments were performed after receiving approval from the Institutional Animal Care and Use Committee of Clinical Research Institute in Seoul National University Hospital, and they complied with the National Research Council Guidelines for the Care and Use of Laboratory Animals. Male Sprague–Dawley rats, 8 weeks old (Daehan Biolink Co, Chungbuk, South Korea), were fed standard pellet feed and given water ad libitum. Animals were anesthetized with ketamine hydrochloride (50 mg kg−1, Yuhan Corp, Bayer, Seoul, Korea) and xylazine (7 mg kg−1, Yuhan Corp, Bayer). After balloon injury, rosiglitazone (1 mg per day) was administered by osmotic pumps (Alzet) for 14 days.
Immunohistochemical staining and terminal deoxynucleotidyl transferase–mediated dUTP nick end labeling (TUNEL) assay
Immunohistochemistry was performed as previously described.10, 11 The primary antibodies used were against proliferating cell nuclear antigen, calponin, α-SMA and thrombospondin. The TUNEL assay was performed with minor modifications to a previously described method, using an Apoptag kit (Intergen Co, Gaithersburg, MD, USA).10, 11 Sections were counterstained with methyl green or Mayer’s hematoxylin.
Statistical analysis
All results are expressed as the mean±s.e. Experimental mean values were compared by Student’s t-test or analysis of variance, as appropriate, using SPSS software version 17.0 (SPSS Inc., Chicago, IL, USA). P<0.05 was considered statistically significant.
Results
Rosiglitazone modulates VSMC phenotype through a PKG-dependent pathway
PDGF treatment changed the morphology of VSMCs from an elongated spindle-shape to a flattened and fibroblast-like shape (Figures 1a and b). Interestingly, rosiglitazone treatment reversed this morphologic change induced by PDGF, resulting in retention of the spindle-shaped morphology (Figure 1c). Moreover, the PKG inhibitor 8-Rp-cPT-cGMP blocked the effect of rosiglitazone (Figure 1d), suggesting that rosiglitazone modulates VSMC morphology via a PKG-dependent pathway. This morphologic difference was quantified by using the ratio of the long axis to the short axis of cells (Figure 1e). To confirm the effect of rosiglitazone on the phenotype of VSMCs, immunofluorescence staining was performed. When treated with PDGF, a contractile phenotype of VSMCs (positive for calponin and α-SMA) was changed to a synthetic one (positive for thrombospondin; Figure 2). Rosiglitazone blocked this effect of PDGF, which was reversed by PKG inhibitor. These results suggest that rosiglitazone modulates VSMC phenotype through a PKG-dependent pathway.
Rosiglitazone (RSG) changes vascular smooth muscle cell (VSMC) morphology via the protein kinase G (PKG) pathway. (a–d) Representative images of morphologic changes of VSMCs treated with various chemicals. Platelet-derived growth factor (PDGF) treatment changed VSMC morphology from a spindle-shaped contractile form to an epitheloid synthetic form. However, RSG treatment blocked the change of VSMC morphology induced by PDGF treatment. In addition, PKG inhibitor (8-Rp-cPT-cGMP) reversed the effect of RSG, suggesting that the effect of RSG on VSMC morphology is mediated through a PKG pathway. Scale bar=100 μm. (e) Quantitative graph of the ratio of pole-to-pole length (long axis/short axis) in each group. *P<0.05 vs vehicle. **P<0.05 vs PDGF+RSG, n=4. Inset figures are magnified forms of original figures.
Rosiglitazone (RSG) modulates vascular smooth muscle cell (VSMC) phenotype through protein kinase G (PKG) activation. Immunofluorescence staining for calponin, α-smooth muscle actin (SMA) and thrombospondin. Calponin and α-SMA are markers of the differentiated contractile form of VSMCs. In contrast, thrombospondin is a marker of the synthetic form of VSMCs. (a, b) Platelet-derived growth factor (PDGF) treatment reduced calponin and α-SMA levels. RSG treatment prevented the change in each marker induced by PDGF treatment. PKG inhibitor reversed the effect of RSG, suggesting that RSG modulated VSMC phenotype through PKG activation. Scale bar=10 μm. (c) The opposite effect was observed for thrombospondin.
Rosiglitazone increases the expression of PKG Iα, but not PKG Iβ
PKG is classified into PKG I and PKG II, and PKG I is also divided into PKG Iα and PKG Iβ. We tested whether rosiglitazone could change the expression levels of these PKG isoforms. PDGF treatment decreased the level of PKG Iα, but not PKG Iβ (Figure 3). Rosiglitazone treatment effectively blocked the decrease of PKG Iα induced by PDGF. These results suggest that rosiglitazone regulates the transcriptional level of PKG Iα.
Rosiglitazone (RSG) increases the expression level of protein kinase G (PKG) Iα, but not PKG Iβ. Western blot showed that platelet-derived growth factor (PDGF) treatment reduced the total amount of PKG I and that this effect of PDGF was blocked by RSG. Interestingly, PDGF treatment changed the level of PKG Iα, but not that of PKG Iβ. These results suggest that RSG regulates the expression of PKG Iα, but not PKG Iβ. n=4.
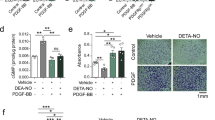
Rosiglitazone modulates VSMC viability via PKG activation
Western blot analysis of phospho-VASP (a substrate of PKG) showed that rosiglitazone treatment increased the activity of PKG (Figure 4a). Moreover, PKG inhibitor reversed the PKG activation induced by rosiglitazone, suggesting that rosiglitazone increased not only the expression level but also the activity of PKG. Interestingly, GW9662, a peroxisome proliferator-activated receptor (PPAR)-γ antagonist, reversed the rosiglitazone-induced phosphorylation of VASP (Figure 4a), suggesting that rosiglitazone modulates PKG activity through a PPARγ-dependent mechanism. Other assays showed that rosiglitazone treatment reduced the proliferation and viability of VSMCs and that PKG inhibitor reversed the effect of rosiglitazone on VSMCs, suggesting that rosiglitazone also changes VSMC viability through PKG activation (Figures 4b and c).
Rosiglitazone (RSG) also increases protein kinase G (PKG) activity and phosphorylated vasodilator-stimulated phosphoprotein (VASP) at serine 239, resulting in the reduction of vascular smooth muscle cell (VSMC) viability. (a) Phosphorylation of VASP (a substrate of PKG) at serine 239 reflects PKG activity and inhibits VSMC proliferation. Western blot analysis showed that RSG treatment increased VASP phosphorylation, which was reversed by PKG inhibitor, suggesting that RSG increased PKG activity. Moreover, GW9662, a peroxisome proliferator-activated receptor (PPAR)-γ antagonist, reversed the increased phosphorylation of VASP induced by RSG. These results suggest that the effect of RSG on PKG activity is mediated through a PPARγ-dependent mechanism. Arrow indicates the phosphorylated form of VASP at serine 239. The right panel indicates the quantitative graph of the western blot analysis in the left upper panel. V, vehicle, pVASP 239, phospho-VASP at serine 239. (b, c) Quantitative graphs of bromodeoxyuridine (BrdU) incorporation assay for VSMC proliferation and cell counting for VSMC viability. RSG treatment reduced the increased proliferation and viability of VSMCs induced by platelet-derived growth factor (PDGF) treatment. This effect was reversed by PKG inhibitor, suggesting that the effect of RSG on VSMC viability was also regulated by PKG activation. *P<0.05 vs vehicle+PDGF. **P<0.05 vs PDGF+RSG, n=4.
The effect of rosiglitazone is mediated not by the change of NO-cGMP but by the increased binding of Sp1 on the PKG promoter
NO upregulates the level of cGMP, which can also activate PKG. Therefore, we examined which part of the NO-cGMP-PKG pathway was modulated by rosiglitazone. Interestingly, rosiglitazone did not change the level of NO or cGMP, suggesting that rosiglitazone directly influences PKG itself without influencing NO or cGMP (Figures 5a–c). ChIP for the PKG promoter showed that rosiglitazone increased the binding of Sp1 on the promoter region of PKG (Figure 5d). Moreover, mithramycin, a Sp1 inhibitor, reversed the increased binding activity of Sp1 induced by rosiglitazone. Taken together, all these results suggest that the effect of rosiglitazone is exerted by the increase of Sp1 binding on the PKG promoter, not by increasing the level of NO-cGMP (Figure 5e).
The activation of PKG induced by rosiglitazone (RSG) is mediated not by a change in NO or cGMP but by the increased binding of Sp1 on the protein kinase G (PKG) promoter. (a–c) We tested the effect of RSG on the NO-cGMP-PKG pathway. RSG did not change the level of NO or cGMP. iNOS, inducible nitric oxide synthase, n=4. (d) Chromatin immunoprecipitation (ChIP) assay for the PKG promoter showed that the activation of PKG was induced by the increased binding of Sp1 on the promoter region of PKG. Moreover, mithramycin, a Sp1 inhibitor, reversed the increased binding activity of Sp1 induced by RSG. (e) Collectively, all these results suggest that the effect of RSG is exerted by the increase of Sp1-binding activity on the PKG promoter, not by regulating the level of NO or cGMP. GAPDH, glyceraldehyde 3-phosphate dehydrogenase; IP, immunoprecipitation; sGC, soluble guanylyl cyclase; V, vehicle; VASP, vasodilator-stimulated phosphoprotein; VSMC, vascular smooth muscle cell.
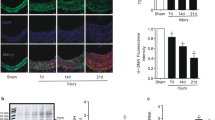
Rosiglitazone reduces neointimal hyperplasia after angioplasty in vivo by modulating VSMC phenotype
Rosiglitazone treatment significantly reduced neointimal formation at 2 weeks after vascular injury in vivo compared with the vehicle-treated group (Figure 6a). Quantitative analysis showed a significant reduction in intima/media ratio (vehicle-treated group vs rosiglitazone-treated group, 0.78±0.2 vs 0.47±0.1, P<0.05, n=10 in each group; Figure 6b). In the injured arteries, rosiglitazone treatment decreased VSMC proliferation and increased VSMC apoptosis (Figures 6c and d). Moreover, rosiglitazone treatment reversed the decreased levels of calponin and α-SMA induced by vascular injury (Figures 6e and f). In contrast, the level of thrombospondin, a marker for synthetic VSMC, was decreased by rosiglitazone treatment (Figure 6g). These results suggest that rosiglitazone could prevent neointimal hyperplasia by modulating the VSMC phenotype even in vivo.
Rosiglitazone (RSG) treatment reduces neointimal hyperplasia after vascular injury in rat carotid artery by modulating vascular smooth muscle cell (VSMC) phenotype. (a) Representative images of the vessels at 2 weeks after injury. RSG treatment significantly reduced neointimal hyperplasia compared with the balloon-injury-alone group. A, adventitia; I, intima; M, media; scale bar=100 μm (upper panel) and 20 μm (lower panel). (b) Quantification graphs of I/M ratio at 2 weeks after injury. This graph shows a markedly reduced I/M ratio in the RSG-treated group (n=10). *P<0.05 vs balloon injury group. (c, d) Immunohistochemical staining for terminal deoxynucleotidyl transferase (TdT)–mediated dUTP nick end labeling (TUNEL) or proliferating cell nuclear antigen (PCNA) expression in the carotid artery wall. Compared with the balloon injury group, RSG treatment decreased VSMC proliferation (PCNA positive) and increased VSMC apoptosis (TUNEL positive). Scale bar=20 μm. (e–g) Immunohistochemical staining for calponin, α-smooth muscle actin (SMA) and thrombospondin. (e, f) Compared with the intact arteries before balloon injury, the injured arteries showed decreased calponin and α-SMA (markers of contractile form), which was reversed by RSG treatment. Scale bar=20 μm. (g) For thrombospondin (a marker of synthetic form), RSG had the opposite effect. These results suggest that RSG could prevent neointimal hyperplasia by modulating VSMC phenotype even in vivo.
Discussion
VSMCs have a critical role in the pathogenesis of vascular disorders such as hypertension and atherosclerosis. In particular, the synthetic or proliferative phenotype of VSMCs contributes to the progression of atherosclerosis or neointimal formation.1 In our study, rosiglitazone modulated VSMC phenotype via the PKG pathway. Rosiglitazone treatment increased the level of PKG, resulting in increased phosphorylation of VASP at Ser239. This phosphorylation of VASP inhibited VSMC proliferation and reduced VSMC viability. Interestingly, rosiglitazone upregulated PKG by increasing the binding activity of Sp1 on the PKG promoter, not by upregulating NO or cGMP, which are upstream of PKG. Moreover, rosiglitazone effectively reduced neointimal formation after vascular injury by modulating the VSMC phenotype in vivo.
PKG has an important role in modulating VSMC phenotype
The phenotype change of VSMCs in response to injury is an important process for atherosclerosis progression and neointimal formation.6, 8 Activation of VSMCs induced by vascular injury converts them from a contractile to a synthetic phenotype. Synthetic VSMCs show high rates of proliferation, migration and production of extracellular matrix components, such as collagen, elastin and proteoglycans, which constitute the atheroma or neointima.12, 13 The phenotype change of VSMCs from a contractile to a synthetic form is associated with a reduced level of PKG.14, 15, 16, 17 Based on this fact, PKG overexpression can reduce restenosis after vascular injury.18 This suggests that PKG has an important role in modulating the VSMC phenotype and neointimal formation, which are associated with restenosis after balloon injury or stent implantation.
Rosiglitazone modulates VSMC phenotype by regulating PKG Iα, but not PKG Iβ
Two forms of PKG, namely PKG I and PKG II, have been identified in mammals.19 PKG I is expressed in VSMCs, platelets, cardiomyocytes, endothelial cells and neuronal cells.19 Moreover, PKG I has two isoforms, PKG Iα and PKG Iβ. In the cardiovascular system, each cell type has a different expression pattern of PKG I isoforms. For example, cardiomyocytes mainly have PKG Iα, but VSMCs have both PKG Iα and PKG Iβ.8 PKG Iα is more easily activated by cGMP than is PKG Iβ.20 The exact roles of PKG Iα and PKG Iβ in vascular biology are unclear. However, each isoform of PKG I seems to have a different role in VSMCs. For example, the dysregulation of Ca2+ related to vasorelaxation is rescued by PKG Iα, not by PKG Iβ.21 In our study, rosiglitazone increased the expression of PKG Iα, but not PKG Iβ. However, besides the transcriptional change of PKG Iα induced by rosiglitazone, our study did not show whether the activity of one or both isoforms of PKG I was affected by rosiglitazone.
The effect of rosiglitazone on VSMC proliferation and viability is mediated by VASP phosphorylation at Ser239
Several mechanisms are associated with the proliferation or apoptosis of VSMCs after vascular injury. Rosiglitazone inhibits VSMC proliferation through a PPARγ pathway or GSK-3β activation.22, 23 In this study, we discovered a new mechanism by which rosiglitazone inhibits VSMC proliferation: activation of PKG. VASP is a substrate of PKG. There are three phosphorylation sites on VASP, serine 157, serine 239 and threonine 278.24 VASP phosphorylation at serine 239 is associated with inhibition of VSMC proliferation, whereas phosphorylation at serine 157 enhances VSMC growth.25 Interestingly, VASP phosphorylation at serine 239 is preferred by PKG.25 Our finding that rosiglitazone increased VASP phosphorylation at serine 239 shows the potent inhibitory effect on the proliferation of VSMCs, resulting in reducing neointimal hyperplasia.
Rosiglitazone increases the expression of PKG by enhancing the binding activity of Sp1 on the PKG promoter
Several transcription factors, such as Sp1, Krüppel-like transcription factor 4, upstream stimulatory factor 1, upstream stimulatory factor 2 and p53, directly regulate PKG expression.26, 27, 28, 29 After testing the effects of rosiglitazone on the levels of the above transcriptional factors (data not shown), we found that rosiglitazone regulated the binding activity of Sp1 on the PKG promoter. Sp1 increases PKG Iα expression, but how Sp1 acts on the PKG promoter is unknown.26 Our ChIP assay showed that rosiglitazone dramatically increased the binding activity of Sp1 on the PKG promoter, whereas PDGF treatment decreased this promoter-binding activity. Mithramycin, a Sp1 inhibitor, reduced the binding activity induced by rosiglitazone, suggesting that the increased activity of PKG transcription by rosiglitazone is mediated by Sp1.
Rosiglitazone modulates PKG activity by a PPARγ-dependent mechanism
When considering the mechanisms of action of a PPARγ agonist, it is important to distinguish whether that action is mediated by PPARγ-dependent or -independent mechanisms. In our study, PPARγ antagonist reversed the effect of rosiglitazone on VASP phosphorylation, suggesting that the change in PKG activity was mediated by a PPARγ-dependent pathway. Although it is possible that the effect of rosiglitazone on VSMC proliferation could be attributed to rosiglitazone itself, at least under our study conditions, the effect of rosiglitazone on the phenotype modulation of VSMCs seems to have been mediated by PKG activation through a PPARγ-dependent mechanism.
Recent concerns about the safety of rosiglitazone in cardiovascular diseases
Recently, in cardiovascular medicine, there have been many concerns about the safety of rosiglitazone. However, rosiglitazone exerts no significant harmful effects on the heart itself.3, 30, 31 Because PPARγ agonists have the property of fluid retention, rosiglitazone may increase intravascular volume, resulting in an increased burden on the heart, followed by poor prognosis.3 However, recent large clinical trials and meta-analysis show that the effect of rosiglitazone on the heart is neutral or marginally beneficial.3, 31 Although rosiglitazone is not allowed to be sold without a prescription from certified doctors in the United States, our study shows the important role of PPARγ in cardiovascular disease.
An emerging protective role of PKG in the cardiovascular system
The PKG pathway is involved in the protection of the cardiovascular system. Takimoto et al. showed that the activation of PKG by sildenafil suppresses cardiac hypertrophy and modulates cardiac remodeling.32 cGMP/PKG pathway also has an important protective role in the process of reperfusion injury after ischemia.33 Particularly, the NO/cGMP/PKG pathway has a strong beneficial effect in endothelial cells.34 Therefore, rosiglitazone could have beneficial effects on various heart diseases through PKG activation.
In conclusion, our study demonstrated that rosiglitazone can modulate VSMC phenotype effectively through a PKG-dependent pathway, resulting in the reduction of neointimal hyperplasia after angioplasty. These results suggest that the phenotypic modulation of VSMCs via PKG activation underlies the cardiovascular-protective effect of rosiglitazone.
References
Ceriello A . Thiazolidinediones as anti-inflammatory and anti-atherogenic agents. Diabetes Metab Res Rev 2008; 24: 14–26.
Khanderia U, Pop-Busui R, Eagle KA . Thiazolidinediones in type 2 diabetes: a cardiology perspective. Ann Pharmacother 2008; 42: 1466–1474.
Krentz A . Thiazolidinediones: effects on the development and progression of type 2 diabetes and associated vascular complications. Diabetes Metab Res Rev 2009; 25: 112–126.
Rizos CV, Liberopoulos EN, Mikhailidis DP, Elisaf MS . Pleiotropic effects of thiazolidinediones. Expert Opin Pharmacother 2008; 9: 1087–1108.
Sarafidis PA . Thiazolidinedione derivatives in diabetes and cardiovascular disease: an update. Fundam Clin Pharmacol 2008; 22: 247–264.
Lin G, Chow S, Lin J, Wang G, Lue TF, Lin CS . Effect of cell passage and density on protein kinase G expression and activation in vascular smooth muscle cells. J Cell Biochem 2004; 92: 104–112.
Yoshida T, Owens GK . Molecular determinants of vascular smooth muscle cell diversity. Circ Res 2005; 96: 280–291.
Munzel T, Feil R, Mulsch A, Lohmann SM, Hofmann F, Walter U . Physiology and pathophysiology of vascular signaling controlled by guanosine 3′,5′-cyclic monophosphate-dependent protein kinase [corrected]. Circulation 2003; 108: 2172–2183.
Sachinidis A, Flesch M, Ko Y, Schror K, Bohm M, Dusing R et al. Thromboxane A2 and vascular smooth muscle cell proliferation. Hypertension 1995; 26: 771–780.
Yang HM, Kim HS, Park KW, You HJ, Jeon SI, Youn SW et al. Celecoxib, a cyclooxygenase-2 inhibitor, reduces neointimal hyperplasia through inhibition of Akt signaling. Circulation 2004; 110: 301–308.
Park KW, Yang HM, Youn SW, Yang HJ, Chae IH, Oh BH et al. Constitutively active glycogen synthase kinase-3beta gene transfer sustains apoptosis, inhibits proliferation of vascular smooth muscle cells, and reduces neointima formation after balloon injury in rats. Arterioscler Thromb Vasc Biol 2003; 23: 1364–1369.
Kawai-Kowase K, Owens GK . Multiple repressor pathways contribute to phenotypic switching of vascular smooth muscle cells. Am J Physiol Cell Physiol 2007; 292: C59–C69.
Owens GK, Kumar MS, Wamhoff BR . Molecular regulation of vascular smooth muscle cell differentiation in development and disease. Physiol Rev 2004; 84: 767–801.
Boerth NJ, Dey NB, Cornwell TL, Lincoln TM . Cyclic GMP-dependent protein kinase regulates vascular smooth muscle cell phenotype. J Vasc Res 1997; 34: 245–259.
Lincoln TM, Dey NB, Boerth NJ, Cornwell TL, Soff GA . Nitric oxide--cyclic GMP pathway regulates vascular smooth muscle cell phenotypic modulation: implications in vascular diseases. Acta Physiol Scand 1998; 164: 507–515.
Dey NB, Foley KF, Lincoln TM, Dostmann WR . Inhibition of cGMP-dependent protein kinase reverses phenotypic modulation of vascular smooth muscle cells. J Cardiovasc Pharmacol 2005; 45: 404–413.
Dey NB, Boerth NJ, Murphy-Ullrich JE, Chang PL, Prince CW, Lincoln TM . Cyclic GMP-dependent protein kinase inhibits osteopontin and thrombospondin production in rat aortic smooth muscle cells. Circ Res 1998; 82: 139–146.
Sinnaeve P, Chiche JD, Gillijns H, Van Pelt N, Wirthlin D, Van De Werf F et al. Overexpression of a constitutively active protein kinase G mutant reduces neointima formation and in-stent restenosis. Circulation 2002; 105: 2911–2916.
Birschmann I, Walter U . Physiology and pathophysiology of vascular signaling controlled by guanosine 3′,5′-cyclic monophosphate-dependent protein kinase. Acta Biochim Pol 2004; 51: 397–404.
Ruth P, Pfeifer A, Kamm S, Klatt P, Dostmann WR, Hofmann F . Identification of the amino acid sequences responsible for high affinity activation of cGMP kinase Ialpha. J Biol Chem 1997; 272: 10522–10528.
Hofmann F, Ammendola A, Schlossmann J . Rising behind NO: cGMP-dependent protein kinases. J Cell Sci 2000; 113: 1671–1676.
Phillips JW, Barringhaus KG, Sanders JM, Yang Z, Chen M, Hesselbacher S et al. Rosiglitazone reduces the accelerated neointima formation after arterial injury in a mouse injury model of type 2 diabetes. Circulation 2003; 108: 1994–1999.
Lee CS, Kwon YW, Yang HM, Kim SH, Kim TY, Hur J et al. New mechanism of rosiglitazone to reduce neointimal hyperplasia: activation of glycogen synthase kinase-3beta followed by inhibition of MMP-9. Arterioscler Thromb Vasc Biol 2009; 29: 472–479.
Deguchi A, Soh JW, Li H, Pamukcu R, Thompson WJ, Weinstein IB . Vasodilator-stimulated phosphoprotein (VASP) phosphorylation provides a biomarker for the action of exisulind and related agents that activate protein kinase G. Mol Cancer Ther 2002; 1: 803–809.
Chen L, Daum G, Chitaley K, Coats SA, Bowen-Pope DF, Eigenthaler M et al. Vasodilator-stimulated phosphoprotein regulates proliferation and growth inhibition by nitric oxide in vascular smooth muscle cells. Arterioscler Thromb Vasc Biol 2004; 24: 1403–1408.
Sellak H, Yang X, Cao X, Cornwell T, Soff GA, Lincoln T . Sp1 transcription factor as a molecular target for nitric oxide-- and cyclic nucleotide--mediated suppression of cGMP-dependent protein kinase-Ialpha expression in vascular smooth muscle cells. Circ Res 2002; 90: 405–412.
Zeng Y, Zhuang S, Gloddek J, Tseng CC, Boss GR, Pilz RB . Regulation of cGMP-dependent protein kinase expression by Rho and Kruppel-like transcription factor-4. J Biol Chem 2006; 281: 16951–16961.
Sellak H, Choi C, Browner N, Lincoln TM . Upstream stimulatory factors (USF-1/USF-2) regulate human cGMP-dependent protein kinase I gene expression in vascular smooth muscle cells. J Biol Chem 2005; 280: 18425–18433.
Tedeschi A, Nguyen T, Steele SU, Feil S, Naumann U, Feil R et al. The tumor suppressor p53 transcriptionally regulates cGKI expression during neuronal maturation and is required for cGMP-dependent growth cone collapse. J Neurosci 2009; 29: 15155–15160.
Schernthaner G . Pleiotropic effects of thiazolidinediones on traditional and non-traditional atherosclerotic risk factors. Int J Clin Pract 2009; 63: 912–929.
Home PD, Pocock SJ, Beck-Nielsen H, Curtis PS, Gomis R, Hanefeld M et al. Rosiglitazone evaluated for cardiovascular outcomes in oral agent combination therapy for type 2 diabetes (RECORD): a multicentre, randomised, open-label trial. Lancet 2009; 373: 2125–2135.
Takimoto E, Champion HC, Li M, Belardi D, Ren S, Rodriguez ER et al. Chronic inhibition of cyclic GMP phosphodiesterase 5 A prevents and reverses cardiac hypertrophy. Nat Med 2005; 11: 214–222.
Garcia-Dorado D, Agullo L, Sartorio CL, Ruiz-Meana M . Myocardial protection against reperfusion injury: the cGMP pathway. Thromb Haemost 2009; 101: 635–642.
Hood J, Granger HJ . Protein kinase G mediates vascular endothelial growth factor-induced Raf-1 activation and proliferation in human endothelial cells. J Biol Chem 1998; 273: 23504–23508.
Acknowledgements
This study was supported by grants from the Bio & Medical Technology Development Program of the National Research Foundation (NRF) funded by the Korean government (2010–0020258) and from the Innovative Research Institute for Cell Therapy (A062260).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Rights and permissions
This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivs 3.0 Unported License. To view a copy of this license, visit http://creativecommons.org/licenses/by-nc-nd/3.0/
About this article
Cite this article
Yang, HM., Kim, BK., Kim, JY. et al. PPARγ modulates vascular smooth muscle cell phenotype via a protein kinase G-dependent pathway and reduces neointimal hyperplasia after vascular injury. Exp Mol Med 45, e65 (2013). https://doi.org/10.1038/emm.2013.112
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/emm.2013.112
Keywords
This article is cited by
-
PPARγ activation but not PPARγ haplodeficiency affects proangiogenic potential of endothelial cells and bone marrow-derived progenitors
Cardiovascular Diabetology (2014)