Abstract
Background/Objectives:
Recent metabolomics technique reveals a plasma-free amino acid (PFAA)-based metabolite signature that is suggestive of altered PFAAs being an early manifestation of obesity-related insulin resistance. However, the PFAA profiles within non-obese, but more insulin-resistant Asians are not well researched. Compared with Caucasians, Asian populations have more central adiposity, which is generally regarded as metabolically more adverse, but the underlying mechanisms remain unclear. In the present study, we examined whether PFAA profiling was at least one important factor mediating central adiposity and insulin resistance, and aid in cardiovascular risk assessment in healthy Asians with normal body weight.
Subject/Methods:
This was a cross-sectional study. A total of 190 healthy men (n=87 with a mean±s.d. body mass index (BMI) of 23.5±3.5 kg/m2) and women (n=103 with a mean±s.d. BMI of 21.4±3.7 kg/m2) residing in Singapore took part in this study. PFAA levels were measured by using an amino acid analyzer. Basic anthropometric measurements, fasting blood glucose, fasting serum insulin and lipid profiles were obtained using standard protocols.
Results:
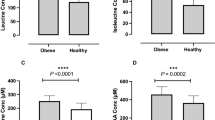
Seven out of 18 amino acids were significantly correlated with measures of obesity (for example, waist circumference; waist-to-hip ratio and BMI) in current participants. Among them, the plasma concentrations of five amino acids, including Phe, Tyr, Met, Ala and His were positively associated with waist-to-hip ratio. With the exception of His, which had no association with insulin resistance, Phe, Tyr, Met and Ala were significantly associated with hyperinsulinemia and insulin resistance (P<0.05). In contrast, no associations were observed between circulating BCAAs (that is, Val, Leu, Ile), measures of obesity and insulin resistance. However, significant inverse associations were observed between BCAAs and total cholesterol and high-density lipoprotein.
Conclusions:
We found that central adiposity was associated with alterations of specific amino acids. As a result, PFAAs may serve as metabolite predictors of hyperglycemia, hyperinsulinemia and dyslipidemia in healthy participants.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout

Similar content being viewed by others
References
Yoon KH, Lee JH, Kim JW, Cho JH, Choi YH, Ko SH et al. Epidemic obesity and type 2 diabetes in Asia. Lancet 2006; 368: 1681–1688.
Ma RCW, Chan JCN . Type 2 diabetes in East Asians: Similarities and differences with populations in Europe and the United States. Ann N Y Acad Sci 2013; 1281: 64–91.
Chiu KC, Cohan P, Lee NP, Chuang LM . Insulin sensitivity differs among ethnic groups with a compensatory response in beta-cell function. Diabetes Care 2000; 23: 1353–1358.
Lear SA, Humphries KH, Kohli S, Chockalingam A, Frohlich JJ, Birmingham CL . Visceral adipose tissue accumulation differs according to ethnic background: results of the Multicultural Community Health Assessment Trial (M-CHAT). Am J Clin Nutr 2007; 86: 353–359.
Choi H, Song S, Kim J, Chung J, Yoon J, Paik HY et al. High carbohydrate intake was inversely associated with high-density lipoprotein cholesterol among Korean adults. Nutr Res 2012; 32: 100–106.
Amati F, Dube JJ, Coen PM, Stefanovic-Racic M, Toledo FGS, Goodpaster BH . Physical inactivity and obesity underlie the insulin resistance of aging. Diabetes Care 2009; 32: 1547–1549.
Ruige JB, Assendelft WJJ, Dekker JM, Kostense PJ, Heine RJ, Bouter LM . Insulin and risk of cardiovascular disease: a meta-analysis. Circulation 1998; 97: 996–1001.
Seibert R, Abbasi F, Hantash FM, Caulfield MP, Reaven G, Kim SH . Relationship between insulin resistance and amino acids in women and men. Physiol Rep 2015; 3: e12392.
Wurtz P, Soininen P, Kangas AJ, Rönnemaa T, Lehtimäki T, Kähönen M et al. Branched-chain and aromatic amino acids are predictors of insulin resistance in young adults. Diabetes Care 2013; 36: 648–655.
Wurtz P, Makinen VP, Soininen P, Kangas AJ, Tukiainen T, Kettunen J et al. Metabolic signatures of insulin resistance in 7098 young adults. Diabetes 2012; 61: 1372–1380.
Yamada C, Kondo M, Kishimoto N, Shibata T, Nagai Y, Imanishi T et al. Association between insulin resistance and plasma amino acid profile in non-diabetic Japanese subjects. J Diabetes Invest 2015; 6: 408–415.
Felig P, Marliss E, Cahill GF Jr . Plasma amino acid levels and insulin secretion in obesity. N Engl J Med 1969; 281: 811–816.
Newgard CB, An J, Bain JR, Muehlbauer MJ, Stevens RD, Lien LF et al. A branched-chain amino acid-related metabolic signature that differentiates obese and lean humans and contributes to insulin resistance. Cell Metab 2009; 9: 311–326.
Lynch CJ, Adams S . Branched-chain amino acids in metabolic signalling and insulin resistance. Nat Rev Endocrinol 2014; 10: 723–736.
Lackey DE, Lynch CJ, Olson KC, Mostaedi R, Ali M, Smith WH et al. Regulation of adipose branched-chain amino acid catabolism enzyme expression and cross-adipose amino acid flux in human obesity. Am J Physiol Endocrinol Metab 2013; 304: E1175–E1187.
Yamakado M, Tanaka T, Nagao K, Ishizaka Y, Mitushima T, Tani M et al. Plasma amino acid profile is associated with visceral fat accumulation in obese Japanese subjects. Clin Obes 2012; 2: 29–40.
Tochikubo O, Nakamura H, Jinzu H, Nagao K, Yoshida H, Kageyama N et al. Weight loss is associated with plasma free amino acid alterations in subjects with metabolic syndrome. Nutr Diabetes 2016; 6: e197.
Wang TJ, Larson MG, Vasan RS, Cheng S, Phee EP, McCabe E et al. Metabolite profiles and the risk of developing diabetes. Nat Med 2011; 17: 448–454.
Floegel A, Stefan N, Yu Z, Muhlenbruch K, Drogan D, Joost HG et al. Identification of serum metabolites associated with risk of type 2 diabetes using a targeted metabolomic approach. Diabetes 2013; 62: 639–648.
Kume S, Araki S, Ono N, Shinhara A, Muramatsu T, Araki H et al. Predictive properties of plasma amino acid profile for cardiovascular disease in patients with type 2 diabetes. PLoS ONE 2015; 9: e101219.
Yamakado M, Nagao K, Imaizumi A, Tani M, Toda A, Tanaka T et al. Plasma free amino acid profiles predict four-year risk of developing diabetes, metabolic syndrome, dyslipidemia, and hypertension in Japanese population. Sci Rep 2015; 5: 11918.
Tai ES, Tan MLS, Stevens RD, Low YL, Muehlbauer MJ, Goh DLM et al. Insulin resistance is associated with a metabolic profile of altered protein metabolism in Chinese and Asian-Indian men. Diabetologia 2010; 53: 757–767.
Huffman KM, Shah SH, Stevens RD . Relationships between circulating metabolic intermediates and insulin action in overweight to obese, inactive men and women. Diabetes Care 2009; 32: 1678–1683.
Bi X, Tey SL, Leong C, Quek R, Loo YT, Henry CJ . Correlation of adiposity indices with cardiovascular disease risk factors in healthy adults of Singapore: a cross-sectional study. BMC Obesity 2016; 3: 33.
Bi X, Tey SL, Leong C, Quek R, Henry CJ . Prevalence of vitamin D deficiency in Singapore: its implications to cardiovascular risk factors. PLoS ONE 2016; 11: e0147616.
Shah SH, Crosslin DR, Haynes CS . Branched-chain amino acid levels are associated with improvement in insulin resistance with weight loss. Diabetologia 2012; 55: 321–330.
McCormack SE, Shaham O, McCarthy MA . Circulating branched-chain amino acid concentrations are associated with obesity and future insulin resistance in children and adolescents. Pediatr Obes 2013; 8: 52–61.
Magnusson M . A diabetes-predictive amino acid score and future cardiovascular disease. Eur Heart J 2013; 34: 1982–1989.
Hattersley JG, Pfeiffer AFH, Roden M, Petzke KJ, Hoffmann D, Rudovich NN et al. Modulation of amino acid metabolic signatures by supplemented isoenergetic diets differing in protein and cereal fiber content. J Clin Endocrinol Metab 2014; 99: E2599–E2609.
Tillin T, Hughes AD, Wang Q . Diabetes risk and amino acid profiles: cross-sectional and prospective analyses of ethnicity, amino acids and diabetes in a South Asian and European cohort from the SABRE (Southall and brent revisited) study. Diabetologia 2015; 58: 968–979.
She P, VanHorn CG, Reid T, Hutson SM . Obesity-related elevations in plasma leucine are associated with defective branched chain amino acid (BCAA) metabolism. FASEB J 2007; 21: 830.3.
Gogna N, Krishna M, Oommen AM, Dorai K . Investigating correlations in the altered metabolic profiles of obese and diabetic subjects in a South Indian Asian population using an NMR-based metabolomic approach. Mol Biosyst 2015; 11: 595–606.
Arner P . Catecholamine-induced lipolysis in obesity. Int J Obes 1999; 23: 10–13.
Fiehn O, Garvey WT, Newman JW, Lok KH, Hoppel CL, Adams SH . Plasma metabolomic profiles reflective of glucose homeostasis in non-diabetic and type 2 diabetic obese African-American women. PLoS One 2010; 5: e15234.
Elshorbagy AK, Nurk E, Gjesdal CG, Tell GS, Ueland PM, Nygard O et al. Homocysteine, cysteine, and body composition in the Hordaland Homocysteine Study: does cysteine link amino acid and lipid metabolism? Am J Clin Nutr 2008; 88: 738–746.
Stone KP, Wanders D, Orgeron M, Cortez CC, Gettys TW . Mechanisms of increased in vivo insulin sensitivity by dietary methionine restriction in mice. Diabetes 2014; 63: 3721–3733.
Holecek M . Relation between glutamine, branched-chain amino acids, and protein metabolism. Nutrition 2002; 18: 130–133.
Nakamura H, Jinzu H, Nagao K, Noguchi Y, Shimba N, Miyano H et al. Plasma amino acid profiles are associated with insulin, C-peptide and adiponectin levels in type 2 diabetic patients. Nutr Diabetes 2014; 4: e133.
Zhang Y, Guo K, LeBlanc RE, Loh D, Schwartz GJ, Yu YH . Increasing dietary leucine intake reduces diet-induced obesity and improves glucose and cholesterol metabolism in mice via multimechanisms. Diabetes 2007; 56: 1647–1654.
Torres-Leal FL, Fonseca-Alaniz MH, Teodoro GFR, Capitani MD, Vianna D, Pantaleao LC et al. Leucine supplementation improves adiponectin and total cholesterol concentrations despite the lack of changes in adiposity or glucose homeostasis in rats previously exposed to a high-fat diet. Nutr Metab 2011; 8: 62.
Ohara M, Doi H, Hayasi M, Satomi S . Administration of L-valine lowered plasma cholesterol by accelerating the conversion of cholesterol into bile acid. Clin Nutr 2003; 22: S57.
Weickert MO, Roden M, Isken F, Hoffmann D, Nowotny P, Osterhoff M et al. Effects of supplemented isoenergetic diets differing in cereal fiber and protein content on insulin sensitivity in overweight humans. Am J Clin Nutr 2011; 94: 459–471.
Shimomura Y, Honda T, Shiraki M, Murakami T, Sato J, Kobayashi H et al. Branched-chain amino acid catabolism in exercise and liver disease. J Nutr 2006; 136: 250S–253S.
Acknowledgements
We greatly acknowledge the financial support from Singapore Institute for Clinical Sciences, Agency for Science, Technology, and Research (A*Star), Singapore. Clinical Trial No: ACTRN12614000643673.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Rights and permissions
About this article
Cite this article
Bi, X., Tey, S., Loo, Y. et al. Central adiposity-induced plasma-free amino acid alterations are associated with increased insulin resistance in healthy Singaporean adults. Eur J Clin Nutr 71, 1080–1087 (2017). https://doi.org/10.1038/ejcn.2017.34
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ejcn.2017.34