Abstract
Background/Objectives:
Zinc is a negative acute-phase reactant; hence, its concentration decreases in the presence of inflammation. There is no current consensus on how to control for the effect of inflammation on serum zinc, which has implications for accurate estimates of population-level zinc status. We aimed to measure the association between inflammation and serum zinc concentrations and to compare the means and the prevalence of zinc deficiency using unadjusted and inflammation-adjusted serum zinc concentrations among Congolese children.
Subjects/Methods:
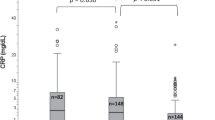
Non-fasting blood was collected in the afternoon in trace element-free vacutainers from 744 apparently healthy children aged 6–59 months in the Democratic Republic of the Congo. Serum was analyzed for zinc, C-reactive protein (CRP) and α-1 acid glycoprotein (AGP) for 665 children with complete data for all three biomarkers. Linear regression was used to generate correction factors (CFs) based on three stages of inflammation: incubation (CRP >5 mg/l and normal AGP), early convalescence (CRP >5 mg/l and AGP >1 g/l) and late convalescence (AGP >1 g/l and normal CRP), relative to no inflammation.
Results:
Overall unadjusted mean±s.d. serum zinc concentration was 9.4±2.1 μmol/l. Study-generated CFs (95% confidence interval) for incubation, early and late convalescence were 1.01 (0.88, 1.14), 1.15 (1.11, 1.21) and 1.07 (1.03, 1.11), respectively. After applying the CFs, overall adjusted mean±s.d. serum zinc concentration was 10.1±2.2 μmol/l, and prevalence of zinc deficiency (<8.7 μmol/l) decreased from 35% (n=234/665) to 24% (n=160/665).
Conclusions:
Adjustment of zinc concentrations for inflammation is warranted when assessing population-level zinc status.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout
Similar content being viewed by others
References
Tomkins A . Assessing micronutrient status in the presence of inflammation. J Nutr 2003; 133: 1649–1655.
Thurnham DI, McCabe LD, Haldar S, Wieringa FT, Northrop-Clewes CA, McCabe GP . Adjusting plasma ferritin concentrations to remove the effects of subclinical inflammation in the assessment of iron deficiency: a meta-analysis. Am J Clin Nutr 2010; 92: 546–555.
Thurnham DI, Northrop-clewes CA, Knowles J . The use of adjustment factors to address the impact of inflammation on vitamin A and iron status in humans. J Nutr 2015; 145: 1137–43S.
Thurnham DI, McCabe GP, Northrop-Clewes CA, Nestel P . Effects of subclinical infection on plasma retinol concentrations and assessment of prevalence of vitamin A deficiency: meta-analysis. Lancet 2003; 362: 2052–2058.
Harvey-Leeson S, Karakochuk CD, Hawes M, Tugirimana PL, Bahizire E, Akilimali PZ et al. Anemia and micronutrient status of women of childbearing age and children 6–59 months in the Democratic Republic of the Congo. Nutrients 2016; 8: 98.
Brown KH, Rivera JA, Bhutta Z, Gibson RS, King JC, Lönnerdal B et al. International Zinc Nutrition Consultative Group (IZiNCG) technical document #1. Assessment of the risk of zinc deficiency in populations and options for its control. Food Nutr Bull 2004; 25 (1 Suppl 2), S99–203.
Smith J, Butrimovitz G . Direct measurement of zinc in plasma by atomic absorption spectroscopy. Clin Chem 1979; 25: 1487–1491.
Erhardt JG, Estes JE, Pfeiffer CM, Biesalski HK, Craft NE . Combined measurement of ferritin, soluble transferrin receptor, retinol binding protein, and C-reactive protein by an inexpensive, sensitive, and simple sandwich enzyme-linked immunosorbent assay technique. J Nutr 2004; 134: 3127–3132.
Cogill B . Anthropometric Indicators Measurement Guide. Food and Nutrition Technical Assistance (FANTA): Washington, DC, USA, 2003.
Ashley EA, White NJ . The duration of plasmodium falciparum infections. Malar J 2014; 13: e500.
Mburu ASW, Thurnham DI, Mwaniki DL, Muniu EM, Alumasa FM . The influence and benefits of controlling for inflammation on plasma ferritin and hemoglobin responses following a multi-micronutrient supplement in apparently healthy, HIV+ Kenyan adults. Eur J Clin Nutr 2010; 64: 510–517.
Kuvibidila S, Vuvu M . Unusual low plasma levels of zinc in non-pregnant Congolese women. Br J Nutr 2009; 101: 1783–1786.
Ferguson EL, Gibson RS, Opare-Obisaw C, Osei-Opare F, Stephen AM, Lehrfeld J et al. The zinc, calcium, copper, manganese, nonstarch polysaccharide and phytate content of seventy-eight locally grown and prepared African foods. J Food Compos Anal 1993; 6: 87–99.
Manary MJ, Abrams SA, Griffin IJ, Quimper MM, Shulman RJ, Hamzo MG et al. Perturbed zinc homeostasis in rural 3-5-y-old Malawian children is associated with abnormalities in intestinal permeability attributed to tropical enteropathy. Pediatr Res 2010; 67: 671–675.
Arsenault JE, Wuehler SE, de Romaña DL, Penny ME, Sempértegui F, Brown KH . The time of day and the interval since previous meal are associated with plasma zinc concentrations and affect estimated risk of zinc deficiency in young children in Peru and Ecuador. Eur J Clin Nutr 2011; 65: 184–190.
Acknowledgements
We thank Tze Lin Chai, Jen Foley, Kyly Whitfield and Sarah Harvey, The University of British Columbia for their contributions to this work; Juergen Erhardt, Willstaett, Germany, for conducting the inflammation biomarker analyses, and Karl Bailey, University of Otago, Dunedin, New Zealand for conducting the serum zinc analyses. This research was supported by HarvestPlus, a challenge program of the Consultative Group on International Agricultural Research (CGIAR) Research Program on Agriculture for Nutrition and Health (grant number 2014H8307). This study was conducted according to the guidelines laid down in the Declaration of Helsinki and all procedures involving human subjects/patients were approved by the Clinical Research Ethics Board at the University of British Columbia (H14-01279), the Université de Kinshasa (ESP/CE/033/14) and the Université Catholique de Bukavu (UCB/CIE/NC/25/2014). Written informed consent was obtained from all subjects.
Author contributions
CDK designed the research, conducted the data analysis and drafted the research manuscript; SIB, E Boy, E Bahizire, PLT, PZA, LAH and TJG contributed to the preparation of the manuscript; CDK had primary responsibility for the final content. All authors read and approved the manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest. HarvestPlus contributed to the study design, but did not have a role in the analysis of samples or data, or the final interpretation of findings.
Rights and permissions
About this article
Cite this article
Karakochuk, C., Barr, S., Boy, E. et al. The effect of inflammation on serum zinc concentrations and the prevalence estimates of population-level zinc status among Congolese children aged 6–59 months. Eur J Clin Nutr 71, 1467–1470 (2017). https://doi.org/10.1038/ejcn.2017.127
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ejcn.2017.127