Abstract
Background:
Down syndrome (DS) is the most common human chromosomal abnormality. It is characterized by mental retardation and several metabolic disturbances, including elevated oxidative stress, which may be causally linked. Treatment with dietary antioxidants has been suggested as a potential method to alleviate the oxidative damage and retardation of DS patients, but prior supplementation work has been equivocal.
Aim:
To evaluate the effects of supplementation with antioxidants α-tocopherol and α-lipoic acid (ALA) on oxidative stress biomarkers in DS children.
Methods:
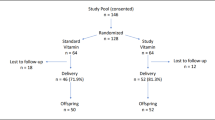
Ninety-three DS children aged 7–15 years from both sexes were randomly allocated to three groups: α-tocopherol (400 IU/day), ALA (100 mg/day) and placebo. The intervention period was 4 months. A healthy control group consisted 26 non-DS siblings. Serum thiobarbituric acid reactive substances (TBARS) and urinary 8-hydroxy-2′-deoxyguanosine (8OHdG) were used as biomarkers of oxidative stress.
Results:
DS children had greater levels of baseline oxidative stress than their siblings. Moreover, males had greater levels of 8OHdG than females (P<0.001) but there was no significant association between age and biomarkers of oxidative stress. Serum levels of TBARS did not change significantly over time, or relative to placebo. Although urinary 8OHdG concentrations decreased significantly in both α-tocopherol and ALA, groups compared with the baseline levels (P<0.001), mean final levels of urinary 8OHdG concentrations differed significantly only between α-tocopherol and placebo groups (P<0.01).
Conclusions:
α-Tocopherol supplementation of the diets of DS children may attenuate oxidative stress at the DNA level.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Iles RK . Cell and molecular biology, and genetic disorders. In Kumar P, Clark M. (eds) Clinical Medicine. 5th edn. W. B. Saunders: Edinburgh, UK, 2004, pp 172–173.
Mégarbané A, Ravel A, Mircher C, Sturtz F, Grattau Y, Rethoré MO et al. The 50th anniversary of the discovery of trisomy 21: the past, present, and future of research and treatment of Down syndrome. Genet Med 2009; 11: 611–616.
de Haan JB, Susil B, Pritchard M . An altered antioxidant balance occurs in Down syndrome fetal organs. J Neural Transm Suppl 2003; 67: 67–83.
Nagy Z . Mechanisms of neuronal death in Down's syndrome. J Neural Transm suppl 1999; 57: 223–245.
Muchova J, Sustrova M . Influence of age on activities of antioxidant enzymes and lipid peroxidation products in erythrocytes and neutrophils of Down syndrome patients. Free Radical Biol Med 2001; 31: 499–508.
Nachvak SM, Mahboob SA, Speakman JR . Low consumption of fruit and vegetables, and markers of oxidative stress in children with Down syndrome. Downs Syndr Res Pract 2010; 13.
Ellis JM, Tan HK, Gilbert RE, Muller DP, Henley W, Moy R et al. Supplementation with antioxidants and folinic acid for children with Down's syndrome: randomised controlled trial. BMJ 2008; 336: 594–597.
Lott IT, Doran E, Nguyen VQ, Tournay A, Head E, Gillen DL . Down syndrome and dementia: a randomized, controlled trial of antioxidant supplementation. Am J Med Genet A 2011; 155A: 1939–1948.
Gallagher ML . The nutrients and their metabolism. Mahan LK, Escott Stump S (eds). Krause's Food and the Nutrition Care Process. 13th edn Saunders: Pennsylvania, USA, 2012, pp 32–125.
Satoh K . Serum lipid peroxide in cerebrovascular disorders determined by new colorimetric method. Clinical Chimica Acta 1978; 90: 37–13.
Thompson HJ, Heimendinger J, Haegele A, Sedlacek SM, Gillette C, O'Neill C et al. Effect of increased vegetable and fruit consumption on markers of oxidative cellular damage. Carcinogenesis 1999; 20: 2261–2266.
Guzmán R, Campos C, López-Fernández E, Casado A . Biomarkers of age effect on renal function in Down syndrome. Biomarkers 2011; 16: 679–685.
Jovanovic SV, Clements D, MacLeod K . Biomarkers of oxidative stress are significantly elevated in Down syndrome. Free Radic Biol Med 1998; 25: 1044–1048.
Zitnanová I, Korytár P, Aruoma OI, Sustrová M, Garaiová I, Muchová J et al. Uric acid and allantoin in Down syndrome: antioxidant and oxidative stress mechanism. Clin Chim Acta 2004; 341: 139–146.
Carratelli M, Porcaro L, Ruscica M, De Simone E, Bertelli AA, Corsi MM . Reactive oxygen metabolites and pro-oxidant status in children with Down's syndrome. Int J Clin Pharmacol Res. 2001; 21: 79–84.
Pinto M, Neves J, Palha M, Bicho M . Oxidative stress in Portuguese children with Down syndrome. Downs Syndr Res Prac 2002; 8: 79–82.
Garces ME, Peres W, Salvador M . Oxidative stress and hematologic and biochemical parameters in individuals with Down syndrome. Mayo Clin Proc 2005; 80: 1607–1611.
Midorikawa K, Kawanishi S . Superoxide dismutases enhance H2O2-induced DNA damage and alter its site specificity. FEBS Lett. 2001; 495: 187–190.
Goldstein S, Meyerstein D, Czapski G . The Fenton reagents. Free Radic Biol Med 1993; 15: 435–445.
Sinha S . Anti-oxidant gene expression imbalance, aging and Down syndrome. Life Sci 2005; 76: 1407–1426.
Meagher EA, FitzGerald GA . Indices of lipid peroxidation in vivo: strengths and limitations. Free Radic Biol Med 2000; 28: 1745–1750.
Kosugi H, Kato T, Kikugawa K . Formation of yellow, orange and red pigments in the reaction of alk-2-enals with 2-thiobarbituric acid. Anal Biochem 1987; 165: 456–464.
Gutteridge HTW . Aspects to consider when detecting and measuring lipid peroxidation. Free Radic Res Commun 1986; 1: 173–184.
Rossner P, Sram RJ . Immunochemical detection of oxidatively damaged DNA. Free Radic Res 2012; 46: 492–522.
Breton J, Sichel F, Bianchini F, Prevost V . Measurement of 8-hydroxy-2’-deoxyguanosine by a commercially available ELISA test: comparison with HPLC/electrochemical detection in calf thymus DNA and determination in human serum. Anal Lett 2003; 36: 123–134.
Yin B, Whyatt RM, Perera FP, Randall MC, Jedrychowski W, Cooper Y, Santella RM . Determination of 8-hydroxydeoxyguanosine by immunoaffinity chromatography-monoclonal antibody based ELISA. Free Radic Biol Med 1995; 18: 1023–1032.
Hu CW, Wu MT, Chao MR, Pan CH, Wang CJ, Swenberg JA et al. Comparison of analyses of urinary 9-hydroxy-2’-deoxyguanosine by isotope dilution liquid chromatography with electrosprat tandem mass spectrometry and by enzyme-linked immunosorbent assay. Rapid Commun Mass Sp 2004; 18: 505–510.
Song MF, Li YS, Ootsuyama Y, Kasai H, Kawai K, Ohta M et al. Urea, the most abundant component in urine, cross-reacts with a commercial 8-OH-dG ELISA kit and contributes to over-estimation of urinary 8-OH-dG. Free Radic Biol Med 2009; 47: 41–46.
Evans MD, Olinski R, Loft S . Toward consensus in the analysis of urinary 8-oxo 7,8-dihydro-2’-deoxyguanosine as a non-invasive biomarker of oxidative stress. FASEB J 2010; 24: 1249–1260.
Miller ER, Pastor-Barriuso R, Dalal D, Riemersma RA, Appel LJ, Guallar E . Meta-analysis: high-dosage vitamin E supplementation may increase all-cause mortality. Ann Intern Med 2005; 142: 37–46.
Roberts LJ, Oates JA, Linton MF, Fazio S, Meador BP, Gross MD et al. The relationship between dose of vitamin E and suppression of oxidative stress in humans. Free Radic Biol Med 2007; 43: 1388–1393.
Acknowledgements
We thank the families and children involved in our study. We also thank Dr Ahmad Reza Dorosti, Dr Reza Mahdavi, Dr Majid Hajifaraji, Dr Mehdi Hedaiati, Dr Mansoor Rezaei and Mrs Nasrin Shariatzadeh for their valuable contributions. This study was supported in part by National Nutrition and Food Technology Research Institute (NNFTRI). The α-tocopherol was a generous unrestricted gift from DSM Ltd (Netherlands). The trial was given ethical approval by the Ethics committee of the Tehran University of Medical Sciences and registered with the Iranian Clinical Trials Registry (registration number IRCT2013102215111N1).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Supplementary Information accompanies this paper on European Journal of Clinical Nutrition website
Supplementary information
Rights and permissions
About this article
Cite this article
Mustafa Nachvak, S., Reza Neyestani, T., Ali Mahboob, S. et al. α-Tocopherol supplementation reduces biomarkers of oxidative stress in children with Down syndrome: a randomized controlled trial. Eur J Clin Nutr 68, 1119–1123 (2014). https://doi.org/10.1038/ejcn.2014.97
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ejcn.2014.97
This article is cited by
-
The Role of Oxidative Stress in Trisomy 21 Phenotype
Cellular and Molecular Neurobiology (2023)