Abstract
Background/objective:
Artificial sweeteners were thought to be metabolically inactive, but after demonstrating that the gustatory mechanism was also localized in the small intestine, suspicions about the metabolic effects of artificial sweeteners have emerged. The objective of this study was to determine the effect of artificial sweeteners (aspartame and sucralose) on blood glucose, insulin, c-peptide and glucagon-like peptide-1 (GLP-1) levels.
Subjects/methods:
Eight newly diagnosed drug-naive type 2 diabetic patients (mean age 51.5±9.2 years; F/M: 4/4) and eight healthy subjects (mean age 45.0±4.1 years; F/M: 4/4) underwent 75 g oral glucose tolerance test (OGTT). During OGTT, glucose, insulin, c-peptide and GLP-1 were measured at 15- min intervals for 120 min. The OGTTs were performed at three settings on different days, where subjects were given 72 mg of aspartame and 24 mg of sucralose in 200 ml of water or 200 ml of water alone 15 min before OGTT in a single-blinded randomized order.
Results:
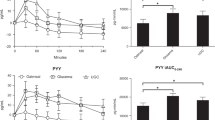
In healthy subjects, the total area under the curve (AUC) of glucose was statistically significantly lower in the sucralose setting than in the water setting (P=0.002). There was no difference between the aspartame setting and the water setting (P=0.53). Total AUC of insulin and c-peptide was similar in aspartame, sucralose and water settings. Total AUC of GLP-1 was significantly higher in the sucralose setting than in the water setting (P=0.04). Total AUC values of glucose, insulin, c-peptide and GLP-1 were not statistically different in three settings in type 2 diabetic patients.
Conclusions:
Sucralose enhances GLP-1 release and lowers blood glucose in the presence of carbohydrate in healthy subjects but not in newly diagnosed type 2 diabetic patients.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
Höfer D, Asan E, Drenckhahn D . Chemosensory perception in the gut. News Physiol Sci 1999; 14: 18–23.
Höfer D, Püschel B, Drenckhahn D . Taste receptor-like cells in the rat gut identified by expression of alpha-gustducin. Proc Natl Acad Sci USA 1996; 93: 6631–6634.
Jang HJ, Kokrashvili Z, Theodorakis MJ, Carlson OD, Kim BJ, Zhou J et al. Gut-expressed gustducin and taste receptors regulate secretion of glucagon-like peptide-1. Proc Natl Acad Sci USA 2007; 104: 15069–15074.
Kokrashvili Z, Mosinger B, Margolskee RF . Taste signaling elements expressed in gut enteroendocrine cells regulate nutrient-responsive secretion of gut hormones. Am J Clin Nutr 2009; 90: 822S–825S.
Margolskee RF, Dyer J, Kokrashvili Z, Salmon KS, Ilegems E, Daly K et al. T1R3 and gustducin in gut sense sugars to regulate expression of Na+-glucose cotransporter 1. Proc Natl Acad Sci USA 2007; 104: 15075–15080.
Hellen M . Sweeteners and Sugar Alternatives in Food Tecnology, 1st edn. Blackwell Publishing Ltd: Garsington Road, Oxford, UK, 2006, pp 146–148.
Brown RJ, Walter M, Rother KI . Effects of diet soda on gut hormones in youths with diabetes. Diabetes Care 2012; 35: 959–964.
Reimann F, Habib AM, Tolhurst G, Parker HE, Rogers GJ, Gribble FM . Glucose sensing in L cells: a primary cell study. Cell Metab 2008; 8: 532–539.
Steinert RE, Frey F, Töpfer A, Drewe J, Beglinger C . Effects of carbohydrate sugars and artificial sweeteners on appetite and the secretion of gastrointestinal satiety peptides. Br J Nutr 2011; 105: 1320–1328.
Ma J, Chang J, Checklin HL, Young RL, Jones KL, Horowitz M et al. Effect of the artificial sweetener, sucralose, on small intestinal glucose absorption in healthy human subjects. Br J Nutr 2010; 104: 803–806.
Ma J, Bellon M, Wishart JM, Young R, Blackshaw LA, Jones KL et al. Effect of the artificial sweetener, sucralose, on gastric emptying and incretin hormone release in healthy subjects. Am J Physiol Gastrointest Liver Physiol 2009; 296: G735–G739.
Gregersen S, Jeppesen PB, Holst JJ, Hermansen K . Antihyperglycemic effects of stevioside in type 2 diabetic subjects. Metabolism 2004; 53: 73–76.
Mezitis NH, Maggio CA, Koch P, Quddoos A, Allison DB, Pi-Sunyer FX . Glycemic effect of a single high oral dose of the novel sweetener sucralose in patients with diabetes. Diabetes Care 1996; 19: 1004–1005.
Wu T, Bound MJ, Standfield SD, Bellon M, Young RL, Jones KL et al. Artificial sweeteners have no effect on gastrc emtying, glucagon-like peptide-1, or glycemia after oral glucose in healthy humans. Diabetes Care 2013; 36: e202–e203.
Grotz VL, Henry RR, McGill JB, Prince MJ, Shamoon H, Trout JR et al. Lack of effect of sucralose on glucose homeostasis in subjects with type 2 diabetes. J Am Diet Assoc 2003; 103: 1607–1612.
Barriocanal LA, Palacios M, Benitez G, Benitez S, Jimenez JT, Jimenez N et al. Apparent lack of pharmacological effect of steviol glycosides used as sweeteners in humans. A pilot study of repeated exposures in some normotensive and ypotensive individuals and in type 1 and type 2 diabetics. Regul Toxicol Pharmacol 2008; 51: 37–41.
Maki KC, Curry LL, Reeves MS, Toth PD, McKenney JM, Farmer MV et al. Chronic consumption of rebaudioside A, a steviol glycoside, in men and women with type 2 diabetes mellitus. Food Chem Toxicol 2008; 46 (Suppl 7): S47–S53.
Cooper PL, Wahlqvist ML, Simpson RW . Sucrose versus saccharin as an added sweetener in non-insulin-dependent diabetes: short- and medium-term metabolic effects. Diabet Med 1988; 5: 676–680.
Pepino MY, Tiemann CD, Patterson BW, Wice BM, Klein S . Sucralose affects glycemic and hormonal responses to an oral glucose load. Diabetes Care 2013; 36: 2530–2535.
Mace OJ, Affleck J, Patel N, Kellett GL . Sweet taste receptors in rat small intestine stimulate glucose absorption through apical GLUT2. J Physiol 2007; 582 (Part 1): 379–392.
Gouyon F, Caillaud L, Carriere V, Klein C, Dalet V, Citadelle D et al. Simple-sugar meals target GLUT2 at enterocyte apical membranes to improve sugar absorption: a study in GLUT2-null mice. J Physiol 2003; 552 (Part 3): 823–832.
Dyer J, Vayro S, King TP, Shirazi-Beechey SP . Glucose sensing in the intestinal epithelium. Eur J Biochem 2003; 270: 3377–3388.
Prigeon RL, Quddusi S, Paty B, D'Alessio DA . Suppression of glucose production by GLP-1 independent of islet hormones: a novel extrapancreatic effect. Am J Physiol Endocrinol Metab 2003; 285: E701–E707.
Vollmer K, Holst JJ, Baller B, Ellrichmann M, Nauck MA, Schmidt WE et al. Predictors of incretin concentrations in subjects with normal, impaired, and diabetic glucose tolerance. Diabetes 2008; 57: 678–687.
Young RL, Sutherland K, Pezos N, Brierley SM, Horowitz M, Rayner CK et al. Expression of taste molecules in the upper gastrointestinal tract in humans with and without type 2 diabetes. Gut 2009; 58: 337–346.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Rights and permissions
About this article
Cite this article
Temizkan, S., Deyneli, O., Yasar, M. et al. Sucralose enhances GLP-1 release and lowers blood glucose in the presence of carbohydrate in healthy subjects but not in patients with type 2 diabetes. Eur J Clin Nutr 69, 162–166 (2015). https://doi.org/10.1038/ejcn.2014.208
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ejcn.2014.208
This article is cited by
-
A high sucrose detection threshold is associated with increased energy intake and improved post-prandial glucose response independent of the sweetness intensity of isocaloric sucrose solutions
npj Metabolic Health and Disease (2024)
-
An alternative pathway for sweet sensation: possible mechanisms and physiological relevance
Pflügers Archiv - European Journal of Physiology (2020)
-
Effects of Non-nutritive Sweeteners on Sweet Taste Processing and Neuroendocrine Regulation of Eating Behavior
Current Nutrition Reports (2020)
-
Non-nutritive Sweeteners and Glycaemic Control
Current Atherosclerosis Reports (2019)