Abstract
Background/objective:
Anorexia nervosa (AN) is a severe eating disorder with a high mortality rate. Treatment regimes show regional and global variation and are sometimes supported by enteral feeding (EF) via nasogastric tube, although risks and benefits are still unclear. We aimed to find out whether EF improves growth and AN recovery and prevents psychiatric comorbidities.
Subjects/methods:
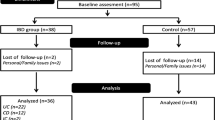
Data were retrospectively collected from medical records and follow-up data were collected via questionnaires. Two hundred and eight female AN patients who were hospitalized below the age of 18 years with a mean follow-up of 6 years were analyzed. We calculated relative risks for the association between EF and suboptimal growth, remission of AN and the occurrence of psychiatric comorbidities, adjusting for potential confounders.
Results:
A third of the analyzed girls received EF at any time. In the adjusted analyses, we found no significant associations between EF and suboptimal growth, the persistence of AN and the occurrence of psychiatric comorbidities, respectively.
Conclusion:
Our data suggest EF to be neither a risk factor nor beneficial for growth, recovery or persistence of AN and the occurrence of psychiatric comorbidities.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout

Similar content being viewed by others
References
The World Health Organization. ICD-10 Classification of Mental and Behavioural Disorders Geneva1993. Available from http://www.who.int/classifications/icd/en/GRNBOOK.pdf.
Harris EC, Barraclough B . Excess mortality of mental disorder. Br J Psychiatry 1998; 173: 11–53.
Signorini A, De Filippo E, Panico S, De Caprio C, Pasanisi F, Contaldo F . Long-term mortality in anorexia nervosa: a report after an 8-year follow-up and a review of the most recent literature. Eur J Clin Nutr 2007; 61: 119–122.
Golden NH, Kreitzer P, Jacobson MS, Chasalow FI, Schebendach J, Freedman SM et al. Disturbances in growth hormone secretion and action in adolescents with anorexia nervosa. J Pediatr 1994; 125: 655–660.
Swenne I, Thurfjell B . Clinical onset and diagnosis of eating disorders in premenarcheal girls is preceded by inadequate weight gain and growth retardation. Acta Paediatr 2003; 92: 1133–1137.
Steinhausen HC . The outcome of anorexia nervosa in the 20th century. Am J Psychiatry 2002; 159: 1284–1293.
Eisler I, Simic M, Russell GF, Dare C . A randomised controlled treatment trial of two forms of family therapy in adolescent anorexia nervosa: a five-year follow-up. J Child Psychol Psychiatry 2007; 48: 552–560.
Lock J, Agras WS, Bryson S, Kraemer HC . A comparison of short- and long-term family therapy for adolescent anorexia nervosa. J Am Acad Child Adolesc Psychiatry 2005; 44: 632–639.
Diamanti A, Basso MS, Castro M, Bianco G, Ciacco E, Calce A et al. Clinical efficacy and safety of parenteral nutrition in adolescent girls with anorexia nervosa. J Adolesc Health 2008; 42: 111–118.
Rigaud D, Brondel L, Poupard AT, Talonneau I, Brun JM . A randomized trial on the efficacy of a 2-month tube feeding regimen in anorexia nervosa: A 1-year follow-up study. Clin Nutr 2007; 26: 421–429.
Serfaty M, McCluskey S . Compulsory treatment of anorexia nervosa and the moribund patient. Eur Eat Disord Rev 1998; 6: 27–37.
Thiels C . Forced treatment of patients with anorexia. Curr Opin Psychiatry 2008; 21: 495–498.
Cockfield A, Philpot U . Feeding size 0: the challenges of anorexia nervosa. Managing anorexia from a dietitian's perspective. Proc Nutr Soc 2009; 68: 281–288.
Herpertz S, Hagenah U, Vocks S, von Wietersheim J, Cuntz U, Zeeck A . The diagnosis and treatment of eating disorders. Dtsch Arztebl Int 2011; 108: 678–685.
Zuercher JN, Cumella EJ, Woods BK, Eberly M, Carr JK . Efficacy of voluntary nasogastric tube feeding in female inpatients with anorexia nervosa. JPEN J Parenter Enteral Nutr 2003; 27: 268–276.
Gueguen J, Godart N, Chambry J, Brun-Eberentz A, Foulon C, Divac Phd SM et al. Severe anorexia nervosa in men: comparison with severe AN in women and analysis of mortality. Int J Eat Disord 2012; 45: 537–545.
Fichter MM, Quadflieg N . Comparing self- and expert rating: a self-report screening version (SIAB-S) of the structured interview for anorexic and bulimic syndromes for DSM-IV and ICD-10 (SIAB-EX). Eur Arch Psychiatry Clin Neurosci 2000; 250: 175–185.
American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders DSM IV 4th edn American Psychiatric Association, 2000.
Derogatis LR, Lipman RS, Rickels K, Uhlenhuth EH, Covi L . The Hopkins Symptom Checklist (HSCL): a self-report symptom inventory. Behav Sci 1974; 19: 1–15.
Rosario AS, Schienkiewitz A, Neuhauser H . German height references for children aged 0 to under 18 years compared to WHO and CDC growth charts. Ann Hum Biol 2011; 38: 121–130.
Kromeyer-Hauschild K, Wabitsch M, Kunze D, Geller F, Geiß HC, Hesse V et al. Perzentile für den Body-mass-Index für das Kindes- und Jugendalter unter Heranziehung verschiedener deutscher Stichproben. Monatsschrift Kinderheilkunde 2001; 149: 807–818.
Rosenbaum PR, Rubin DB . Reducing bias in observational studies using subclassification on the propensity score. J Am Stat Assoc 1984; 79: 516–524.
Robb AS, Silber TJ, Orrell-Valente JK, Valadez-Meltzer A, Ellis N, Dadson MJ et al. Supplemental nocturnal nasogastric refeeding for better short-term outcome in hospitalized adolescent girls with anorexia nervosa. Am J Psychiatry 2002; 159: 1347–1353.
Herpertz-Dahlmann B, Muller B, Herpertz S, Heussen N, Hebebrand J, Remschmidt H . Prospective 10-year follow-up in adolescent anorexia nervosa—course, outcome, psychiatric comorbidity, and psychosocial adaptation. J Child Psychol Psychiatry 2001; 42: 603–612.
Rastam M, Gillberg C, Wentz E . Outcome of teenage-onset anorexia nervosa in a Swedish community-based sample. Eur Child Adolesc Psychiatry 2003; 12: I78–I90.
Fichter MM, Quadflieg N, Hedlund S . Twelve-year course and outcome predictors of anorexia nervosa. Int J Eat Disord 2006; 39: 87–100.
Merrill RM, Richardson JS . Validity of self-reported height, weight, and body mass index: findings from the National Health and Nutrition Examination Survey, 2001-2006. Prev Chronic Dis 2009; 6: A121.
Meyer C, Arcelus J, Wright S . Accuracy of self-reported weight and height among women with eating disorders: a replication and extension study. Eur Eat Disord Rev 2009; 17: 366–370.
Acknowledgements
We are grateful to the support of Dr med. Günther Hinrichs (ZIP Kiel), Dr med. Joachim Walter (Katholisches Kinderkrankenhaus Wilhelmstift, Hamburg), Dr med. Jan Gerrit Behrens (MediClin Seepark, Bad Bodenteich) and Dr phil. Dipl. Psych. Dorothé Verbeek (UKSH Lübeck) who gave clinical advice and made the data available. We thank all patients who took part in this study. We thank Kristiane Krüger for her help in recruiting the sample and collecting the data and to Sabine Brehm, the medical documentalist. We furthermore thank Lucia Albers and Dr Otmar Bayer for statistical support. Parts of the manuscript arose from the PhD Thesis of KK (at the University of Lübeck) and from the Master Thesis of IN (at the University of Munich).
Author Contributions
The author’s responsibilities were as follows: IN: analyses and principle authorship of the manuscript; KK: recruitment and data collection, descriptive analyses; RvK: interpretation of the data and contribution to final draft; UT: conception of research question, project management, contribution to final draft of the manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Supplementary Information accompanies this paper on European Journal of Clinical Nutrition website
Supplementary information
Rights and permissions
About this article
Cite this article
Nehring, I., Kewitz, K., von Kries, R. et al. Long-term effects of enteral feeding on growth and mental health in adolescents with anorexia nervosa—results of a retrospective German cohort study. Eur J Clin Nutr 68, 171–177 (2014). https://doi.org/10.1038/ejcn.2013.244
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ejcn.2013.244
Keywords
This article is cited by
-
Monitoring and treating hypoglycemia during meal-based rapid nutritional rehabilitation in patients with extreme anorexia nervosa
Eating and Weight Disorders - Studies on Anorexia, Bulimia and Obesity (2022)
-
Psychiatric and medical comorbidities of eating disorders: findings from a rapid review of the literature
Journal of Eating Disorders (2022)
-
A systematic review of enteral feeding by nasogastric tube in young people with eating disorders
Journal of Eating Disorders (2021)
-
The use of enteral nutrition in the treatment of eating disorders: a systematic review
Eating and Weight Disorders - Studies on Anorexia, Bulimia and Obesity (2019)