Abstract
Background/objectives:
To evaluate whether undernutrition risk measured using the Spinal Nutrition Screening Tool (SNST) and the Malnutrition Universal Screening Tool (MUST) is associated with worse clinical outcomes in respect of length of in-patient hospital stay (LOS) and mortality in the 12 months after admission to a spinal cord injuries (SCIs) centre.
Methods:
A multicentre, prospective, cross-sectional observational study was conducted in four UK SCI centres (SCICs). A total of 150 SCI patients (aged 18–88 years (median: 44 years), 30.7% females) were studied between July 2009 and March 2010. LOS and mortality 12 months after admission to the SCIC was monitored. Multivariate regression analysis was used to identify unique predictors of the variance of LOS.
Results:
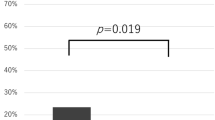
The patients initially undernourished or at risk of undernutrition (44.6%) had a significantly longer LOS (median (days): 129 vs 85, P=0.012) and greater 12-month mortality (% deceased: 9.2% vs 1.4%, P=0.036). In addition, serum albumin and new admission to an SCIC were identified as independent predictors for long LOS.
Conclusion:
The present study suggests that undernutrition risk, as identified by the SNST, is associated with adverse clinical outcomes. Nutritional screening should be helpful in improving clinical outcomes if it promotes more appropriate and effective nutritional intervention.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
Wong S, Derry F, Jamous A, Hirani SP, Grimble G, Forbes A . The prevalence of malnutrition in spinal cord injured patients - a UK multicentre study. Br J Nutr 2012; 108: 918–923.
Wong S, Graham A, Hirani SP, Grimble G, Forbes A . Profile and prevalence of malnutrition in children with spinal cord injuries - assessment of the Screening Tool for Assessment in Paediatrics (STAMP). Spinal Cord 2012; 50: 67–71.
Schneider SM, Veyres P, Pivot X, Soummer AM, Jambou P, Filippi J et al. Malnutrition is an independent factor associated with noscomial infections. Br J Nutr 2004; 92: 105–111.
Ho CH, Powell HL, Collins JF, Bauman WA, Spungen AM . Poor nutrition is a relative contraindication to negative pressure wound therapy for pressure ulcers: preliminary observations in patients with spinal cord injury. Adv Skin Wound Care 2010; 23: 508–516.
National Institute of Health and Clinical Excellence. The management of pressure ulcers in primary and secondary care: A clinical practice guideline. NICE: London, UK, 2005.
Sorensen J, Kondrup J, Prokopowicz J, Schiesser M, Krahenbihl L, Meier R et al. EuroOOPS: an international, multicentre study to implement nutritional risk screening and evaluate clinical outcome. Clin Nutr 2008; 27: 340–349.
National Institute of Health and Clinical Excellence. Nutrition support in adults: oral nutrition support, enteral tube feeding and parenteral nutrition. NICE: London, UK, 2006.
Royal College of Physicians. Chronic Spinal Cord Injury: management of patients in acute hospital setting: National Guidelines. Royal College of Physicians: London, UK, 2008.
Elia M . Screening for Malnutrition: A multidisciplinary Responsibility. Development and Use of the Malnutrition Universal Screening Tool (MUST) for adults. BAPEN: Redditch, UK, 2003.
Kruizenga HM, Seidell JC, de Vet HCW, Wierdsma NJ, van Bokhorst-de van der Schueren MA . Development and validation of a hospital screening tool for malnutrition: the short nutritional assessment questionnaire (SNAQ). Clin Nutr 2005; 24: 75–82.
Morgan MY, Madden AM, Soulsby CT, Morris RW . Derivation and validation of a new global method for assessing nutritional status in patients with cirrhosis. Hepatology 2006; 44: 823–835.
Wong S, Derry F, Jamous A, Hirani SP, Grimble G, Forbes A . Validation of the Spinal Nutrition Screening Tool (SNST) in patients with spinal cord injuries (SCI) - result form a multicentre study. Eur J Clin Nutr 2012; 66: 382–387.
Stratton RJ, Green CJ, Elia M . Disease-related Malnutrition: an evidence-based approach to treatment. CABI Publishing: Oxford, 2003.
Correia MI, Waitzberg DL . The impact of malnutrition on morbidity, mortality, length of hospital stay and costs evaluated through a multivariate model analysis. Clin Nutr 2003; 22: 235–239.
Daverat P, Gagon M, Dartigues JF, Mazaus JM, Barat M . Initial factors predicting survival in patients with a spinal cord injury. J Neurol Neurosurg Psychiatry 1989; 52: 403–406.
Burr RG, Clift-Peace L, Nuseibeh I . Haemoglobin and albumin as predictors of length of stay of spinal injured patients in a rehabilitation centre. Paraplegia 1993; 31: 473–478.
Naber TH, Schermer T, de Bree A, Nusteling K, Eggink L, Kruimel JW et al. Prevalence of malnutrition in nonsurgical hospitalised patients and its association with disease complication. Am J Clin Nutr 1997; 66: 1232–1239.
Hennessey DB, Burke JP, Ni-Dhonochu T, Shields C, Winter DC, Mealy K . Preoperative hypoalbuminemia is an independent risk factor for the development of surgical site infection following gastrointestinal surgery. Ann Surg 2010; 252: 325–329.
Franch-Arcas G . The meaning of hypoalbuminaemia in clinical practice. Clin Nutr 2001; 20: 265–269.
Joint Standard Development Groups of the South England Review Group. Standard for patients requiring spinal cord injury care (Revised 2010). http://www.sci-link.org.uk/wp-content/uploads/2010/09/Standards_SCI_final_May_20101.pdf. Accessed 20 November 2010.
Wong S, Derry F, Grimble G, Forbes A . How do spinal cord injury centre manage malnutrition? A cross-sectional survey of 12 regional centres in the United Kingdom and Ireland. Spinal Cord 2012; 50: 132–135.
Acknowledgements
We are pleased to acknowledge the support from the UK National Institute for Health Research (NIHR) via its funding of the University College London (UCL) Biomedical Research Centre. We would like to thank the patients and ward staff for participating in the study, Dr Allison Graham for reviewing the manuscript and Pauline Bateman at the National Spinal Injuries Centre at Stoke Mandeville Hospital. We would like to thank the staff of different SCI centres for their kind support in the different hospitals participating in the study. The study group included Dr Joan Gandy, The British Dietetic Association; Anthony Twist, The Robert Jones and Agnes Hunt Orthopaedics and District Hospital, Oswestry, UK; Philippa Bearne, Salisbury District Hospital, Salisbury, UK; and Dr Angela Gall and Judith Susser, Royal National Orthopaedic Hospital, Stanmore, UK. We are also grateful to Abbott Nutrition for providing an unrestricted grant to fund this study. The sponsors of the study did not have any role in the study design, data collection, analysis and interpretation, writing of the paper or in the decision to submit the paper for publications.
Author contributions
SW contributed to protocol development, data collection, data analysis, manuscript preparation. FD helped in clinical supervision, manuscript revision. AJ provided clinical supervision, manuscript revision. SPH contributed to statistical supervision, manuscript revision. SPH helped in statistical supervision, manuscript revision. AF contributed to academic supervision, manuscript revision and guarantor. All authors have read and approved the final version submitted for publication.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Part of the study result was presented at the International Spinal Cord Society annual meeting in London, September 2012, and at the European Society of Parenteral and Enteral Nutrition annual meeting in Barcelona, September 2012.
Rights and permissions
About this article
Cite this article
Wong, S., Derry, F., Jamous, A. et al. Is undernutrition risk associated with an adverse clinical outcome in spinal cord-injured patients admitted to a spinal centre?. Eur J Clin Nutr 68, 125–130 (2014). https://doi.org/10.1038/ejcn.2013.238
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ejcn.2013.238
Keywords
This article is cited by
-
Nutritional blood parameters and nutritional risk screening in patients with spinal cord injury and deep pressure ulcer—a retrospective chart analysis
Spinal Cord (2018)
-
Detecting malnutrition risk and obesity after spinal cord injury: a quality improvement project and systematic review
European Journal of Clinical Nutrition (2018)
-
Special considerations in the urological management of the older spinal cord injury patient
World Journal of Urology (2018)
-
Review of dietetic service provision and activity in spinal cord injury centres: a multicentre survey in the UK and Republic of Ireland
Spinal Cord (2015)