Abstract
Background/Objectives:
This study investigates the effects of a carbohydrate (CHO; lotus-root starch) predominant, late-evening snack (LES), containing 200 kcal (50 g CHO) on fasting resting energy expenditure (REE) and nutrient oxidation in hospitalized adults with acute-on-chronic liver failure (ACLF).
Subjects/Methods:
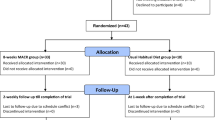
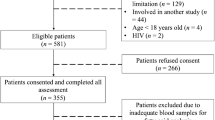
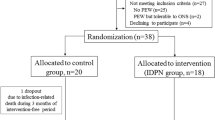
Adults with ACLF were randomized to receive daily LES (treatment; n=35) or standard care (n=35; non-supplemented control) for 14 days. REE and respiratory quotient (RQ) were measured by indirect calorimetry, nutrient oxidation (CHO, protein and fat), intake and biochemical parameters were measured in both groups at baseline and after 14 days using validated techniques. Disease severity was measured using the model for end-stage liver disease (MELD).
Results:
No significant differences in macronutrient intake, anthropometric, demographic characteristics or MELD scores were observed between groups at baseline (P>0.05). Fasting RQ was significantly higher in the LES supplemented verses the control group after 2 weeks (P=0.02). CHO oxidation was significantly higher (P=0.001) and fat oxidation (P=0.02) was lower in the LES-supplemented group when compared with controls after 2 weeks. Fasting RQ and REE in the LES-supplemented group increased significantly (0.83 verses 0.88; P=0.007/1301±409 vs 1687±718 kcal/day; P=0.02) in patients with MELD scores ⩽30 when compared with patients with MELD scores >30 (0.82 verses 0.84; P=0.27/ 1361±405 vs 1437±429 kcal/day; P=0.67) after supplementation.
Conclusions:
A carbohydrate-predominant LES is associated with increases in fasting carbohydrate oxidation, REE and reductions in fat oxidation in adults with ACLF. Therapeutic strategies utilizing LES may promote improved nutritional status in adults with ACLF.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout

Similar content being viewed by others
References
Polson J, Lee WM . AASLD position paper: the management of acute liver failure. Hepatology 2005; 41: 1179–1197.
Liang X, Bi S, Yang W, Wang L, Cui G, Cui F et al. Epidemiological serosurvey of hepatitis B in China—declining HBV prevalence due to hepatitis B vaccination. Vaccine 2009; 27: 6550–6557.
Fan HL, Yang PS, Chen HW, Chen TW, Chan DC, Chu CH et al. Predictors of the outcomes of acute-on-chronic hepatitis B liver failure. World J Gastroenterol 2012; 18: 5078–5083.
Meng QH, Hou W, Yu HW, Lu J, Li J, Wang JH et al. Resting energy expenditure and substrate metabolism in patients with acute-on-chronic hepatitis B liver failure. J Clin Gastroenterol 2011; 45: 456–461.
Meng QH, Wang JH, Yu HW, Li J, Feng YM, Hou W et al. Resting energy expenditure and substrate metabolism in Chinese patients with acute or chronic hepatitis B or liver cirrhosis. Intern Med 2010; 49: 2085–2091.
Meng QH, Yu HW, Li J, Wang JH, Ni MM, Feng YM et al. Inadequate nutritional intake and protein-energy malnutrition involved in acute and chronic viral hepatitis Chinese patients especially in cirrhosis patients. Hepatogastroenterology 2010; 57: 845–851.
Tsien CD, McCullough AJ, Dasarathy S . Late-evening snack: exploiting a period of anabolic opportunity in cirrhosis. J Gastroenterol Hepatol 2012; 27: 430–441.
Arabi Y, Ahmed QA, Haddad S, Aljumah A, Al-Shimemeri A . Outcome predictors of cirrhosis patients admitted to the intensive care unit. Eur J Gastroenterol Hepatol 2004; 16: 333–339.
Fan CL, Wu YJ, Duan ZP, Zhang B, Dong PL, Ding HG . Resting energy expenditure and glucose, protein and fat oxidation in severe chronic virus hepatitis B patients. World J Gastroenterol 2008; 14: 4365–4369.
Bankhead R, Boullata J, Brantley S, Corkins M, Guenter P, Krenitsky J et al. Enteral nutrition practice recommendations. JPEN J Parenter Enteral Nutr 2009; 33: 122–167.
Plauth M, Schuetz T . Hepatology—Guidelines on Parenteral Nutrition, Chapter 16. Ger Med Sci 2009; 7: Doc12.
Nakaya Y, Okita K, Suzuki K, Moriwaki H, Kato A, Miwa Y et al. BCAA-enriched snack improves nutritional state of cirrhosis. Nutrition 2007; 23: 113–120.
Yamanaka-Okumura H, Nakamura T, Takeuchi H, Miyake H, Katayama T, Arai H et al. Effect of late evening snack with rice ball on energy metabolism in liver cirrhosis. Eur J Clin Nutr 2006; 60: 1067–1072.
Fukushima H, Miwa Y, Ida E, Kuriyama S, Toda K, Shimomura Y et al. Nocturnal branched-chain amino acid administration improves protein metabolism in patients with liver cirrhosis: comparison with daytime administration. JPEN J Parenter Enteral Nutr 2003; 27: 315–322.
Yamauchi M, Takeda K, Sakamoto K, Ohata M, Toda G . Effect of oral branched chain amino acid supplementation in the late evening on the nutritional state of patients with liver cirrhosis. Hepatol Res 2001; 21: 199–204.
Zillikens MC, van den Berg JW, Wattimena JL, Rietveld T, Swart GR . Nocturnal oral glucose supplementation. The effects on protein metabolism in cirrhotic patients and in healthy controls. J Hepatol 1993; 17: 377–383.
Miwa Y, Shiraki M, Kato M, Tajika M, Mohri H, Murakami N et al. Improvement of fuel metabolism by nocturnal energy supplementation in patients with liver cirrhosis. Hepatol Res 2000; 18: 184–189.
Tsuchiya M, Sakaida I, Okamoto M, Okita K . The effect of a late evening snack in patients with liver cirrhosis. Hepatol Res 2005; 31: 95–103.
Okamoto M, Sakaida I, Tsuchiya M, Suzuki C, Okita K . Effect of a late evening snack on the blood glucose level and energy metabolism in patients with liver cirrhosis. Hepatol Res 2003; 27: 45–50.
Sarin SK, Kumar A, Almeida JA, Chawla YK, Fan ST, Garg H et al. Acute-on-chronic liver failure: consensus recommendations of the Asian Pacific Association for the study of the liver (APASL). Hepatol Int 2009; 3: 269–282.
Yang X . China food composition tables. In Yang Yuexin WY, Pan Xingchang eds. China Food Composition Tables 2nd ed Peking University Medical Press: Beijing, China, 2004.
Jyothi AN, Sheriff JT, Sajeev MS . Physical and functional properties of arrowroot starch extrudates. J Food Sci 2009; 74: E97–E104.
Harris JA, Benedict FGA . Biometric Study of Human Basal Metabolism. Proc Natl Acad Sci USA 1918; 4: 370–373.
Weir JB . New methods for calculating metabolic rate with special reference to protein metabolism. J Physiol 1949; 109: 1–9.
Owen OE, Reichle FA, Mozzoli MA, Kreulen T, Patel MS, Elfenbein IB et al. Hepatic, gut, and renal substrate flux rates in patients with hepatic cirrhosis. J Clin Invest 1981; 68: 240–252.
Lusk G . The elements of the science of nutrition. 4th edn, Philadelphia, Pennsylvannia, USA, WB Saunders Co, 1928.
Wiesner R, Edwards E, Freeman R, Harper A, Kim R, Kamath P et al. Model for end-stage liver disease (MELD) and allocation of donor livers. Gastroenterology 2003; 124: 91–96.
Botta F, Giannini E, Romagnoli P, Fasoli A, Malfatti F, Chiarbonello B et al. MELD scoring system is useful for predicting prognosis in patients with liver cirrhosis and is correlated with residual liver function: a European study. Gut 2003; 52: 134–139.
McCullough AJ, Bugianesi E . Protein-calorie malnutrition and the etiology of cirrhosis. Am J Gastroenterol 1997; 92: 734–738.
Koreeda C, Seki T, Okazaki K, Ha-Kawa SK, Sawada S . Effects of late evening snack including branched-chain amino acid on the function of hepatic parenchymal cells in patients with liver cirrhosis. Hepatol Res 2011; 41: 417–422.
Kawamura E, Habu D, Morikawa H, Enomoto M, Kawabe J, Tamori A et al. A randomized pilot trial of oral branched-chain amino acids in early cirrhosis: validation using prognostic markers for pre-liver transplant status. Liver Transpl 2009; 15: 790–797.
Okabayashi T, Nishimori I, Yamashita K, Sugimoto T, Namikawa T, Maeda H et al. Preoperative oral supplementation with carbohydrate and branched-chain amino acid-enriched nutrient improves insulin resistance in patients undergoing a hepatectomy: a randomized clinical trial using an artificial pancreas. Amino Acids 2010; 38: 901–907.
Muto Y, Sato S, Watanabe A, Moriwaki H, Suzuki K, Kato A et al. Effects of oral branched-chain amino acid granules on event-free survival in patients with liver cirrhosis. Clin Gastroenterol Hepatol 2005; 3: 705–713.
Moriwaki H, Shiraki M, Fukushima H, Shimizu M, Iwasa J, Naiki T et al. Long-term outcome of branched-chain amino acid treatment in patients with liver cirrhosis. Hepatol Res 2008; 38: S102–S106.
Marchesini G, Bianchi G, Merli M, Amodio P, Panella C, Loguercio C et al. Nutritional supplementation with branched-chain amino acids in advanced cirrhosis: a double-blind, randomized trial. Gastroenterology 2003; 124: 1792–1801.
Swart GR, Zillikens MC, van Vuure JK, van den Berg JW . Effect of a late evening meal on nitrogen balance in patients with cirrhosis of the liver. Bmj 1989; 299: 1202–1203.
Plank LD, Gane EJ, Peng S, Muthu C, Mathur S, Gillanders L et al. Nocturnal nutritional supplementation improves total body protein status of patients with liver cirrhosis: a randomized 12-month trial. Hepatology 2008; 48: 557–566.
Marchesini G, Marzocchi R, Noia M, Bianchi G . Branched-chain amino Acid supplementation in patients with liver diseases. J Nutr 2005; 135: 1596S–1601S.
Korenaga K, Korenaga M, Uchida K, Yamasaki T, Sakaida I . Effects of a late evening snack combined with alpha-glucosidase inhibitor on liver cirrhosis. Hepatol Res 2008; 38: 1087–1097.
Acknowledgements
We acknowledge the participation of the patients in this study. Funding for this study was provided from the Foundation of Capital Medicine Development Committee (H020920020890). This work was supported by grants from the Foundation of Capital Medicine Development Committee (H020920020890).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Rights and permissions
About this article
Cite this article
Hou, W., Li, J., Lu, J. et al. Effect of a carbohydrate-containing late-evening snack on energy metabolism and fasting substrate utilization in adults with acute-on-chronic liver failure due to Hepatitis B. Eur J Clin Nutr 67, 1251–1256 (2013). https://doi.org/10.1038/ejcn.2013.163
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ejcn.2013.163
Keywords
This article is cited by
-
Management of acute-on-chronic liver failure: an algorithmic approach
Hepatology International (2018)
-
Acute-on-chronic liver failure: terminology, mechanisms and management
Nature Reviews Gastroenterology & Hepatology (2016)
-
Acute-on-chronic Liver Failure
Current Gastroenterology Reports (2016)