Abstract
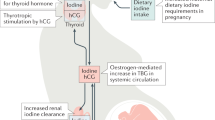
Iodine is required for the production of thyroid hormone. Thyroid hormone affects many metabolic processes in the body, including maturation of the central nervous system. In early pregnancy, the fetus is dependent on maternal thyroid hormone for normal brain development. If iodine deficiency leads to inadequate production of thyroid hormone during pregnancy, irreversible brain damage can result in the fetus. Therefore, achieving adequate iodine nutrition during pregnancy is an important public health objective. Although there have been tremendous gains over the last several decades in our understanding of the effects of iodine deficiency in pregnancy and how to combat them, a number of questions remain about how best to monitor the iodine status of pregnant populations, the effects of mild to moderate iodine deficiency on maternal and child outcomes, the safe upper limit of daily iodine intake in pregnant women and the risks and benefits of iodine supplementation for mildly iodine-deficient pregnant women.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout
Similar content being viewed by others
References
De Escobar GM, Obregon MJ, Del Rey FE . Iodine deficiency and brain development in the first half of pregnancy. Public Health Nutr 2007; 10: 1554–1570.
Younes-Rapozo V, Berendonk J, Savignon T, Manhães AC, Barradas PC . Thyroid hormone deficiency changes the distribution of oligodendrocyte/myelin markers during oligodendroglial differentiation in vitro. Int J Dev Neurosci 2006; 24: 445–453.
Glinoer D . The importance of iodine nutrition during pregnancy. Public Health Nutr 2007; 10: 1542–1546.
Glinoer D . Pregnancy and iodine. Thyroid 2001; 11: 471–481.
Dafnis E, Sabatini S . The effect of pregnancy on renal function: physiology and pathophysiology. Am J Med Sci 1992; 303: 184–205.
World Health Organization. United Nations Children’s Fund, and International Council for the Control of Iodine Deficiency Disorders. Assessment of Iodine Deficiency Disorders and Monitoring their Elimination. A Guide for Programme Managers. 3rd Edition, World Health Organization: Geneva, Switzerland, 2007.
Institute of Medicine. Dietary Reference Intakes for Vitamin A, vitamin K, Arsenic, Boron, Chromium, Copper, Iodine, Iron, Manganese, Molybdenum, Nickel, Silicon, Vanadium, and Zinc. National Academy Press: Washington DC, 2001.
Yoshimura M, Hershman JM . Thyrotropic action of human chorionic gonadotropin. Thyroid 1995; 5: 425–434.
Glinoer D, de Nayer P, Bourdoux P, Lemone M, Robyn C, van Steirteghem A et al. Regulation of maternal thyroid during pregnancy. J Clin Endocrinol Metab 1990; 71: 276–287.
Smyth PPA, Hetherton AM, Ryan R, O’Herlihy C . Alterations in iodine status and thyroid volume in pregnancy. In: Beckers C, Reinwein D (eds) The Thyroid and Pregnancy. Schattauer, Stuttgart: New York, 1991, pp 55–58.
Brander A, Kivisaari L . Ultrasonography of the thyroid during pregnancy. J Clin Ultrasound 1989; 17: 403–406.
Rasmussen NG, Hornnes PJ, Hegedus L . Ultrasonographically determined thyroid size in pregnancy and post partum: the goitrogenic effect of pregnancy. Am J Obstet Gynecol 1989; 160: 1216–1220.
Zoeller TR, Dowling AL, Herzig CT, Iannacone EA, Gauger KJ, Bansal R . Thyroid hormone, brain development, and the environment. Environ Health Perspect 2002; 110: 355–361.
Chen ZP, Hetzel BS . Cretinism revisited. Best Pract Res Clin Endocrinol Metab 2010; 24: 39–50.
Zimmermann MB . Iodine deficiency. Endocr Rev 2009; 30: 376–408.
Qian M, Wang D, Wakins WE, Gebski V, Yan YQ, Li M et al. The effects of iodine on intelligence in children: a meta-anylsis of studies conducted in China. Asia Pac J Clin Nutr 2005; 14: 32–42.
Haddow JE, Palomake GE, Allan WC, Williams JR, Knight GJ, Gagnon J et al. Maternal thyroid deficiency during pregnancy and subsequent neuropsychological development of the child. N Engl J Med 1999; 341: 549–555.
Pop VJ, Kuijpens Jl, van Baar AL, Verkerk G, Verkerk G, van Son MM et al. Low maternal free thyroxine conentrations during early pregnancy are associated with impaired psychomotor development in infancy. Clin Endocrinol 1999; 50: 147–148.
Henrichs J, Bongers-Schokking JJ, Schenk JJ, Ghassabian A, Schmidt HG, Visser TJ . Maternal thyroid function during early pregnancy and cognitive functioning in early childhood: the generation R study. J Clin Endocrinol Metab 2010; 95: 4227–4234.
Wayne EJ, Koutras DA, Alexander WD . Excretion of iodine. In: Clinical Aspects of Iodine Metabolism. Blackwell: Philadelphia, PA: Oxford, 1964, pp 73–83.
Zimmermann MB, Jooste PL, Pandav CS . Iodine-deficiency disorders. Lancet 2008; 372: 1251–1262.
Vought RL, London WT . Iodine intake, excretion and thyroidal accumulation in healthy subjects. J Clin Endocrinol Metab 1967; 27: 913–919.
Rasmussen LB, Ovesen L, Christiansen E . Day-to-day and within-day variation in urinary iodine excretion. Eur J Clin Nutr 1999; 53: 401–407.
König F, Andersson M, Hotz K, Aeberli I, Zimmermann MB . Ten repeat collections for urinary iodine from spot samples or 24 h samples are needed to reliably estimate individual iodine status in women. J Nutr 2011; 141: 2049–2054.
Wong EM, Sullivan KM, Perrine CG, Rogers LM, Peña-Rosas JP . Comparison of median urinary iodine concentration as an indicator of iodine status among pregnant women, school-age children, and nonpregnant women. Food Nutr Bull 2011; 32: 206–212.
Zimmermann MB, Hess SY, Molinari L, De Benoist B, Delange F, Braverman LE et al. New reference values for thyroid volume by ultrasound in iodine-sufficient schoolchildren: a World Health Organization/Nutrition for health and development iodine deficiency study group report. Am J Clin Nutr 2004; 79: 231–237.
Zimmermann MB, de Benoist B, Corigliano S, Jooste PL, Molinari L, Moosa K et al. Assessment of iodine status using dried blood spot thyroglobulin: development of reference material and establishment of an international reference range in iodine-sufficient children. J Clin Endocrinol Metab 2006; 91: 4881–4887.
Skeaff SA . Assessing iodine intakes in pregnancy and strategies for improvement. J Trace Elem Med Biol 2012; 26: 141–144.
Pharoah PO, Connolly KJ . A controlled trial of iodinated oil for the prevention of endemic cretinism: a long-term follow-up. Int J Epidemiol 1987; 16: 68–73.
Zimmermann MB . Iodine deficiency in pregnancy and the effects of maternal iodine supplementation on the offspring: a review. Am J Clin Nutr 2009; 89: 668S–672S.
Romano R, Jannini EA, Pepe M, Grimaldi A, Olivieri M, Spennati P et al. The effects of iodoprophylaxis on thyroid size during pregnancy. Am J Obstet Gynecol 1991; 164: 482–485.
Pedersen KM, Laurberg P, Iversen E, Knudsen PR, Gregersen HE, Rasmussen OS et al. Amelioration of some pregnancy-associated variations in thyroid function by iodine supplementation. J Clin Endocrinol Metab 1993; 77: 1078–1083.
Glinoer D, De Nayer P, Delange F, Lemone M, Toppet V, Spehl M et al. A randomized trial for the treatment of mild iodine deficiency during pregnancy: maternal and neonatal effects. J Clin Endocrinol Metab 1995; 80: 258–269.
Liesenkötter KP, Göpel W, Bogner U, Stach B, Grüters A . Earliest prevention of endemic goiter by iodine supplementation during pregnancy. Eur J Endocrinol 1996; 134: 443–448.
Nøhr SB, Laurberg P . Opposite variations in maternal and neonatal thyroid function induced by iodine supplementation during pregnancy. J Clin Endocrinol Metab 2000; 85: 623–627.
Antonangeli L, Maccherini D, Cavaliere R, Di Giulio C, Reinhardt B, Pinchera A et al. Comparison of two different doses of iodide in the prevention of gestational goiter in marginal iodine deficiency: a longitudinal study. Eur J Endocrinol 2002; 147: 29–34.
Berbel P, Mestre JL, Santamaría A, Palazón I, Franco A, Graells M et al. Delayed neurobehavioral development in children born to pregnant women with mild hypothyroxinemia during the first month of gestation: the importance of early iodine supplementation. Thyroid 2009; 19: 511–519.
Velasco I, Carreira M, Santiago P, Muela JA, García-Fuentes E, Sánchez-Muñoz B et al. Effect of iodine prophylaxis during pregnancy on neurocognitive development of children during the first two years of life. J Clin Endocrinol Metab 2009; 94: 3234–3241.
Murcia M, Rebagliato M, Iñiguez C, Lopez-Espinosa MJ, Estarlich M, Plaza B et al. Effect of iodine supplementation during pregnancy on infant neurodevelopment at 1 year of age. Am J Epidemiol 2011; 173: 804–812.
Zimmermann MB, Connolly K, Bozo M, Bridson J, Rohner F, Grimci L . Iodine supplementation improves cognition in iodine-deficient schoolchildren in Albania: a randomized, controlled, double-blind study. Am J Clin Nutr 2006; 83: 108–114.
Gordon RC, Rose MC, Skeaff SA, Gray AR, Morgan KM, Ruffman T . Iodine supplementation improves cognition in mildly iodine-deficient children. Am J Clin Nutr 2009; 90: 1264–1271.
Melse-Boonstra A, Gowachirapant S, Jaiswal N, Winichagoon P, Srinivasan K, Zimmermann MB . Iodine supplementation in pregnancy and its effect on child cognition. J Trace Elem Med Biol 2012; 26: 134–136.
Wolff J, Chaikoff IL . The temporary nature of the inhibitory action of excess iodine on organic iodine synthesis in the normal thyroid. Endocrinol 1949; 45: 504–501.
Eng PH, Cardona GR, Fang SL, Previti M, Alex S, Carrasco N et al. Escape from the acute Wolff-Chaikoff effect is associated wit ha decrease in thyroid sodium/iodide symporter messenger ribonucleic acid and protein. Endocrinology 1999; 140: 3404–3410.
Markou K, Georgopoulos N, Kyriazopoulou V, Vagenakis AG . Iodine-induced hypothyroidism. Thyroid 2001; 11: 501–510.
Bartalena L, Bogazzi F, Braverman LE, Martino E . Effects of amiodarone administration during pregnancy on neonatal thyroid function and subsequent neurodevelopment. J Endocrinol Invest 2001; 24: 116–130.
Connelly KJ, Boston BA, Pearce EN, Sesser D, Snyder D, Braverman LE et al. Congenital hypothyroidism caused by excess prenatal maternal iodine ingestion. J Pediatr 2012; 161: 760–762.
Opinion of the scientific committee on food on the tolerable upper intake level of iodine; 2002. Available from http://ec.europa.eu/food/fs/sc/scf/out146_en.pdf.
Braverman LE . Adequate iodine intake—the good far outweighs the bad. Eur J Endocrinol 1998; 139: 14–15.
Perrine CG, Herrick K, Serdula MK, Sullivan KM . Some subgroups of reproductive age women in the US may be at risk for iodine deficiency. J Nutr 2010; 140: 1489–1494.
Stagnaro-Green A, Abalovich M, Alexander EK, Alexander E, Azizi F, Mestman J et al. Guidelines of the American Thyroid Association for the Diagnosis and Management of Thyroid Disease During Pregnancy and the Postpartum. Thyroid 2011; 21: 1081–1125.
De Groot L, Abalovich M, Alexander EK, Amino N, Barbour L, Cobin RH et al. Management of thyroid dysfunction during pregnancy and postpartum: an Endocrine Society Clinical Practice Guideline. J Clin Endocrinol Metab 2012; 97: 2543–2565.
WHO Secretariat, Andersson M, de Benoist B, Delange F, Zupan J . Prevention and control of iodine deficiency in pregnant and lactating women and in children less than 2-years-old: conclusions and recommendations of the Technical Consultation. Public Health Nutr 2007; 10: 1606–1611.
Zimmermann MB . Iodine supplementation of pregnant women in Europe: a review and recommendations. Eur J Clin Nutr 2004; 58: 979–984.
Leung AM, Pearce EN, Braverman LE . Iodine content of prenatal multivitamins in the US. N Engl J Med 2009; 360: 939–940.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The author declares no conflict of interest.
Rights and permissions
About this article
Cite this article
Pearce, E. Monitoring and effects of iodine deficiency in pregnancy: still an unsolved problem?. Eur J Clin Nutr 67, 481–484 (2013). https://doi.org/10.1038/ejcn.2012.215
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ejcn.2012.215