Abstract
Background/Objective:
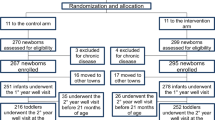
The European Childhood Obesity Project is a multi-centre double-blind randomised clinical trial in five countries testing whether protein intake in early life is related to later obesity risk. We use electronic data capture (EDC) in this trial and report here on our experience with the method.
Subjects/Methods:
Data capture for the first two study years was done with 10 notebooks, which were installed with the RDE (Remote Data Entry) programme Clindoc.
Results:
We here exemplary report our experiences in 1 of the 11 study centres. A total of 205 of 760 visits (27.0%) were documented with interim paper-based case report forms, whereas 555 (73.0%) visits were recorded with electronic case report forms (eCRF). The need for after-trial plausibility checks of anthropometric data is significantly reduced in visits done by eCRF in comparison with visits done by paper-based CRFs (14.05% versus 35.61%).
Conclusion:
EDC reduces significantly the need for after-trial data checks but the planning and implementation process before starting the trial is more time-consuming.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout
Similar content being viewed by others
References
Koletzko B, von Kries R, Closa R, Escribano J, Scaglioni S, Giovannini M et al. Lower protein in infant formula is associated with lower weight up to age 2 y: a randomized clinical trial. Am J Clin Nutr 2009; 89: 1836–1845.
Babre D . Electronic data capture - Narrowing the gap between clinical and data management. Perspect Clin Res 2011; 2: 1–3.
Effler P, Ching-Lee M, Bogard A, Ieong MC, Nedomoto T, Jernigan D . Statewide system of electronic notifiable disease reporting from clinical laboratories: comparing automated reporting with conventional methods. JAMA 1999; 282: 1845–1850.
Bart T . Comparison of Electronic Data Capture with Paper Data Collection - is there really an advantage? In: Balton E (ed.). Business Briefing Pharmatech. Business Briefings Ltd: London, UK, 2003.
Spink C . Electronic Data Capture (EDC) as a means for e-clinical trial success. IBM Global Services, Pharmaceutical Clinical Development 2002.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Rights and permissions
About this article
Cite this article
Pawellek, I., Richardsen, T., Oberle, D. et al. Use of electronic data capture in a clinical trial on infant feeding. Eur J Clin Nutr 66, 1342–1343 (2012). https://doi.org/10.1038/ejcn.2012.141
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ejcn.2012.141