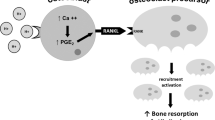
Abstract
High-protein (HP) diets exert a hypercalciuric effect at constant levels of calcium intake, even though the effect may depend on the nature of the dietary protein. Lower urinary pH is also consistently observed for subjects consuming HP diets. The combination of these two effects was suspected to be associated with a dietary environment favorable for demineralization of the skeleton. However, increased calcium excretion due to HP diet does not seem to be linked to impaired calcium balance. In contrast, some data indicate that HP intakes induce an increase of intestinal calcium absorption. Moreover, no clinical data support the hypothesis of a detrimental effect of HP diet on bone health, except in a context of inadequate calcium supply. In addition, HP intake promotes bone growth and retards bone loss and low-protein diet is associated with higher risk of hip fractures. The increase of acid and calcium excretion due to HP diet is also accused of constituting a favorable environment for kidney stones and renal diseases. However, in healthy subjects, no damaging effect of HP diets on kidney has been found in either observational or interventional studies and it seems that HP diets might be deleterious only in patients with preexisting metabolic renal dysfunction. Thus, HP diet does not seem to lead to calcium bone loss, and the role of protein seems to be complex and probably dependent on other dietary factors and the presence of other nutrients in the diet.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout
Similar content being viewed by others
References
Abelow BJ, Holford TR, Insogna KL (1992). Cross-cultural association between dietary animal protein and hip fracture: a hypothesis. Calcif Tissue Int 50, 14–18.
Addis T (1926). The effect of some physiological variables on the number of casts, red blood cells and white blood cells and epithelial cells in the urine of normal individuals. J Clin Invest 2, 417–421.
Alexy U, Remer T, Manz F, Neu CM, Schoenau E (2005). Long-term protein intake and dietary potential renal acid load are associated with bone modeling and remodeling at the proximal radius in healthy children. Am J Clin Nutr 82, 1107–1114.
Allen LH, Bartlett RS, Block GD (1979a). Reduction of renal calcium reabsorption in man by consumption of dietary-protein. J Nutr 109, 1345–1350.
Allen LH, Block GD, Wood RJ, Bryce GF (1981). The role of insulin and parathyroid hormone in the protein-induced calciuria of man. Nutr Res 1, 3–11.
Allen LH, Oddoye EA, Margen S (1979b). Protein-induced hypercalciuria: a longer term study. Am J Clin Nutr 32, 741–749.
Alpern RJ, Sakhaee K (1997). The clinical spectrum of chronic metabolic acidosis: homeostatic mechanisms produce significant morbidity. Am J Kidney Dis 29, 291–302.
Ammann P, Bonjour JP, Rizzoli R (2000). Essential amino acid supplements increase muscle weight, bone mass and bone strength in adult osteoporotic rats. J Musculoskelet Neuronal Interact 1, 43–44.
Anand CR, Linkswiler HM (1974). Effect of protein intake on calcium balance of young men given 500 mg calcium daily. J Nutr 104, 695–700.
Arjmandi BH, Khalil DA, Smith BJ, Lucas EA, Juma S, Payton ME et al. (2003). Soy protein has a greater effect on bone in postmenopausal women not on hormone replacement therapy, as evidenced by reducing bone resorption and urinary calcium excretion. J Clin Endocrinol Metab 88, 1048–1054.
Ball D, Maughan RJ (1997). Blood and urine acid-base status of premenopausal omnivorous and vegetarian women. Br J Nutr 78, 683–693.
Barzel US, Massey LK (1998). Excess dietary protein can adversely affect bone. J Nutr 128, 1051–1053.
Beasley JM, Ichikawa LE, Ange BA, Spangler L, LaCroix AZ, Ott SM et al. (2010). Is protein intake associated with bone mineral density in young women? Am J Clin Nutr 91, 1311–1316.
Bilo HJ, Schaap GH, Blaak E, Gans RO, Oe PL, Donker AJ (1989). Effects of chronic and acute protein administration on renal function in patients with chronic renal insufficiency. Nephron 53, 181–187.
Bonjour JP (2005). Dietary protein: an essential nutrient for bone health. J Am Coll Nutr 24 (6 Suppl), 526S–536S.
Bonjour JP, Schurch MA, Chevalley T, Ammann P, Rizzoli R (1997). Protein intake, IGF-1 and osteoporosis. Osteoporos Int 7 (Suppl 3), S36–S42.
Bourrin S, Ammann P, Bonjour JP, Rizzoli R (2000a). Dietary protein restriction lowers plasma insulin-like growth factor I (IGF-I), impairs cortical bone formation, and induces osteoblastic resistance to IGF-I in adult female rats. Endocrinology 141, 3149–3155.
Bourrin S, Toromanoff A, Ammann P, Bonjour JP, Rizzoli R (2000b). Dietary protein deficiency induces osteoporosis in aged male rats. J Bone Miner Res 15, 1555–1563.
Brenner BM, Meyer TW, Hostetter TH (1982). Dietary protein intake and the progressive nature of kidney disease: the role of hemodynamically mediated glomerular injury in the pathogenesis of progressive glomerular sclerosis in aging, renal ablation, and intrinsic renal disease. N Engl J Med 307, 652–659.
Brinkworth GD, Buckley JD, Noakes M, Clifton PM (2010). Renal function following long-term weight loss in individuals with abdominal obesity on a very-low-carbohydrate diet vs high-carbohydrate diet. J Am Diet Assoc 110, 633–638.
Buclin T, Cosma M, Appenzeller M, Jacquet AF, Decosterd LA, Biollaz J et al. (2001). Diet acids and alkalis influence calcium retention in bone. Osteoporos Int 12, 493–499.
Budek AZ, Hoppe C, Ingstrup H, Michaelsen KF, Bugel S, Molgaard C (2007). Dietary protein intake and bone mineral content in adolescents-The Copenhagen Cohort Study. Osteoporos Int 18, 1661–1667.
Burodom A (2010). Renal response to egg white protein loading in healthy young adults. J Med Assoc Thai 93, 824–829.
Bushinsky DA (1989). Net calcium efflux from live bone during chronic metabolic, but not respiratory, acidosis. Am J Physiol Renal Physiol 256, F836–F842.
Caudarella R, Vescini F, Buffa A, Stefoni S (2003). Citrate and mineral metabolism: kidney stones and bone disease. Front Biosci 8: s1084–s1106.
Ceglia L, Harris SS, Abrams SA, Rasmussen HM, Dallal GE, Dawson-Hughes B (2009). Potassium bicarbonate attenuates the urinary nitrogen excretion that accompanies an increase in dietary protein and may promote calcium absorption. J Clin Endocrinol Metab 94, 645–653.
Chen YM, Teucher B, Tang XY, Dainty JR, Lee KK, Woo JL et al. (2007). Calcium absorption in postmenopausal Chinese women: a randomized crossover intervention study. Br J Nutr 97, 160–166.
Chevalley T, Bonjour JP, Ferrari S, Rizzoli R (2008). High-protein intake enhances the positive impact of physical activity on BMC in prepubertal boys. J Bone Miner Res 23, 131–142.
Collins DM, Rezzo CT, Coffman TM, Klotman PE (1990). Chronic high protein feeding does not reduce glomerular-filtration rate (GFR) in the normal rat. Kidney Int 37, 501–501.
Cooper C, Atkinson EJ, Hensrud DD, Wahner HW, O’Fallon WM, Riggs BL et al. (1996). Dietary protein intake and bone mass in women. Calcif Tissue Int 58, 320–325.
Cummings JH, Hill MJ, Jivraj T, Houston H, Branch WJ, Jenkins DJA (1979). Effect of meat protein and dietary fiber on colonic function ad metabolism .1. Changes in bowel habit, bile-acid excretion, and calcium-absorption. Am J Clin Nutr 32, 2086–2093.
Curhan GC, Willett WC, Knight EL, Stampfer MJ (2004). Dietary factors and the risk of incident kidney stones in younger women: Nurses’ Health Study II. Arch Intern Med 164, 885–891.
Curhan GC, Willett WC, Rimm EB, Stampfer MJ (1993). A prospective study of dietary calcium and other nutrients and the risk of symptomatic kidney stones. N Engl J Med 328, 833–838.
Curhan GC, Willett WC, Speizer FE, Spiegelman D, Stampfer MJ (1997). Comparison of dietary calcium with supplemental calcium and other nutrients as factors affecting the risk for kidney stones in women. Ann Int Med 126, 497–504.
Darling AL, Millward DJ, Torgerson DJ, Hewitt CE, Lanham-New SA (2009). Dietary protein and bone health: a systematic review and meta-analysis. Am J Clin Nutr 90, 1674–1692.
Dawson-Hughes B (2003). Interaction of dietary calcium and protein in bone health in humans. J Nutr 133, 852S–854S.
Dawson-Hughes B, Harris SS (2002). Calcium intake influences the association of protein intake with rates of bone loss in elderly men and women. Am J Clin Nutr 75, 773–779.
Delmi M, Rapin CH, Bengoa JM, Delmas PD, Vasey H, Bonjour JP (1990). Dietary supplementation in elderly patients with fractured neck of the femur. Lancet 335, 1013–1016.
DelValle J, Yamada T (1990). Amino acids and amines stimulate gastrin release from canine antral G-cells via different pathways. J Clin Invest 85, 139–143.
Demigne C, Sabboh H, Remesy C, Meneton P (2004). Protective effects of high dietary potassium: nutritional and metabolic aspects. J Nutr 134, 2903–2906.
Devine A, Criddle RA, Dick IM, Kerr DA, Prince RL (1995). A longitudinal study of the effect of sodium and calcium intakes on regional bone density in postmenopausal women. Am J Clin Nutr 62, 740–745.
Draper HH, Piche LA, Gibson RS (1991). Effects of a high protein intake from common foods on calcium-metabolism in a cohort of postmenopausal women. Nutr Res 11, 273–281.
Erba D, Ciappellano S, Testolin G (2002). Effect of the ratio of casein phosphopeptides to calcium (w/w) on passive calcium transport in the distal small intestine of rats. Nutrition 18, 743–746.
Ettinger B, Pak CYC, Citron JT, Thomas C, AdamsHuet B, Vangessel A (1997). Potassium-magnesium citrate is an effective prophylaxis against recurrent calcium oxalate nephrolithiasis. J Urol 158, 2069–2073.
Fenton TR, Eliasziw M, Lyon AW, Tough SC, Hanley DA (2008). Meta-analysis of the quantity of calcium excretion associated with the net acid excretion of the modern diet under the acid-ash diet hypothesis. Am J Clin Nutr 88, 1159–1166.
Fenton TR, Lyon AW, Eliasziw M, Tough SC, Hanley DA (2009). Meta-analysis of the effect of the acid-ash hypothesis of osteoporosis on calcium balance. J Bone Miner Res 24, 1835–1840.
Ferraretto A, Signorile A, Gravaghi C, Fiorilli A, Tettamanti G (2001). Casein phosphopeptides influence calcium uptake by cultured human intestinal HT-29 tumor cells. J Nutr 131, 1655–1661.
Feskanich D, Willett WC, Stampfer MJ, Colditz GA (1996). Protein consumption and bone fractures in women. Am J Epidemiol 143, 472–479.
Frank H, Graf J, Graf J, Amann-Gassner U, Bratke R, Daniel H et al. (2009). Effect of short-term high-protein compared with normal-protein diets on renal hemodynamics and associated variables in healthy young men. Am J Clin Nutr 90, 1509–1516.
Frassetto L, Morris Jr RC, Sellmeyer DE, Todd K, Sebastian A (2001). Diet, evolution and aging—the pathophysiologic effects of the post-agricultural inversion of the potassium-to-sodium and base-to-chloride ratios in the human diet. Eur J Nutr 40, 200–213.
Frassetto LA, Todd KM, Morris Jr RC, Sebastian A (1998). Estimation of net endogenous noncarbonic acid production in humans from diet potassium and protein contents. Am J Clin Nutr 68, 576–583.
Frassetto LA, Todd KM, Morris Jr RC, Sebastian A (2000). Worldwide incidence of hip fracture in elderly women: relation to consumption of animal and vegetable foods. J Gerontol A Biol Sci Med Sci 55, M585–M592.
Geibel JP, Wagner C (2006). An update on acid secretion. Rev Physiol Biochem Pharmacol 156, 45–60.
Geinoz G, Rapin CH, Rizzoli R, Kraemer R, Buchs B, Slosman D et al. (1993). Relationship between bone mineral density and dietary intakes in the elderly. Osteoporos Int 3, 242–248.
Goss SL, Lemons KA, Kerstetter JE, Bogner RH (2007). Determination of calcium salt solubility with changes in pH and P(CO(2)), simulating varying gastrointestinal environments. J Pharm Pharmacol 59, 1485–1492.
Green J, Kleeman CR (1991). Role of bone in regulation of systemic acid-base balance. Kidney Int 39, 9–26.
Hade JE, Spiro HM (1992). Calcium and acid rebound: a reappraisal. J Clin Gastroenterol 15, 37–44.
Hammond KA, Janes DN (1998). The effects of increased protein intake on kidney size and function. J Exp Biol 201 (Pt 13), 2081–2090.
Hannan MT, Tucker KL, Dawson-Hughes B, Cupples LA, Felson DT, Kiel DP (2000). Effect of dietary protein on bone loss in elderly men and women: the Framingham Osteoporosis Study. J Bone Miner Res 15, 2504–2512.
Hardcastle AC, Aucott L, Reid DM, Macdonald HM (2011). Associations between dietary flavonoid intakes and bone health in a Scottish population. J Bone Miner Res 26, 941–947.
Heaney RP (2000). Dietary protein and phosphorus do not affect calcium absorption. Am J Clin Nutr 72, 758–761.
Heaney RP (2001). Protein intake and bone health: the influence of belief systems on the conduct of nutritional science. Am J Clin Nutr 73, 5–6.
Heaney RP (2002). Protein and calcium: antagonists or synergists? Am J Clin Nutr 75, 609–610.
Heaney RP (2006). Calcium intake and disease prevention. Arq Bras Endocrinol Metabol 50, 685–693.
Heaney RP, Layman DK (2008). Amount and type of protein influences bone health. Am J Clin Nutr 87, 1567S–1570S.
Heaney RP, McCarron DA, Dawson-Hughes B, Oparil S, Berga SL, Stern JS et al. (1999). Dietary changes favorably affect bone remodeling in older adults. J Am Diet Assoc 99, 1228–1233.
Hegsted M, Linkswiler HM (1981). Long-term effects of level of protein intake on calcium metabolism in young adult women. J Nutr 111, 244–251.
Hegsted M, Schuette SA, Zemel MB, Linkswiler HM (1981). Urinary calcium and calcium balance in young men as affected by level of protein and phosphorus intake. J Nutr 111, 553–562.
Hess B (2002). Nutritional aspects of stone disease. Endocrinol Metab Clin North Am 31, 1017–1030, ix and x.
Hirota T, Nara M, Ohguri M, Manago E, Hirota K (1992). Effect of diet and lifestyle on bone mass in Asian young women. Am J Clin Nutr 55, 1168–1173.
Hirvonen T, Pietinen P, Virtanen M, Albanes D, Virtamo J (1999). Nutrient intake and use of beverages and the risk of kidney stones among male smokers. Am J Epidemiol 150, 187–194.
Hu JF, Zhao XH, Parpia B, Campbell TC (1993). Dietary intakes and urinary excretion of calcium and acids: a cross-sectional study of women in China. Am J Clin Nutr 58, 398–406.
Huang Z, Himes JH, McGovern PG (1996). Nutrition and subsequent hip fracture risk among a national cohort of white women. Am J Epidemiol 144, 124–134.
Hunt JR, Gallagher SK, Johnson LK, Lykken GI (1995). High-meat versus low-meat diets–effects on zinc-absorption, iron status, and calcium, copper, iron, magnesium, manganese, nitrogen, phosphorus, and zinc balance in postmenopausal women. Am J Clin Nutr 62, 621–632.
Hunt JR, Johnson LK, Fariba Roughead ZK (2009). Dietary protein and calcium interact to influence calcium retention: a controlled feeding study. Am J Clin Nutr 89, 1357–1365.
Ilich JZ, Brownbill RA, Tamborini L (2003). Bone and nutrition in elderly women: protein, energy, and calcium as main determinants of bone mineral density. Eur J Clin Nutr 57, 554–565.
Ince BA, Anderson EJ, Neer RM (2004). Lowering dietary protein to U.S. Recommended dietary allowance levels reduces urinary calcium excretion and bone resorption in young women. J Clin Endocrinol Metab 89, 3801–3807.
Jaeger P, Bonjour JP, Karlmark B, Stanton B, Kirk RG, Duplinsky T et al. (1983). Influence of acute potassium loading on renal phosphate transport in the rat kidney. Am J Physiol Renal Physiol 245 (5 Pt 1), F601–F605.
Jia Y, Hwang SY, House JD, Ogborn MR, Weiler HA, O K et al. (2010). Long-term high intake of whole proteins results in renal damage in pigs. J Nutr 140, 1646–1652.
Johnson NE, Alcantara EN, Linkswiler H (1970). Effect of level of protein intake on urinary and fecal calcium and calcium retention of young adult males. J Nutr 100, 1425–1430.
Kannus P, Parkkari J, Sievanen H, Heinonen A, Vuori I, Jarvinen M (1996). Epidemiology of hip fractures. Bone 18 (1 Suppl), 57S–63S.
Kerstetter JE, O’Brien KO, Caseria DM, Wall DE, Insogna KL (2005). The impact of dietary protein on calcium absorption and kinetic measures of bone turnover in women. J Clin Endocrinol Metab 90, 26–31.
Kerstetter JE, O’Brien KO, Insogna KL (1998). Dietary protein affects intestinal calcium absorption. Am J Clin Nutr 68, 859–865.
Kerstetter JE, Wall DE, O’Brien KO, Caseria DM, Insogna KL (2006). Meat and soy protein affect calcium homeostasis in healthy women. J Nutr 136, 1890–1895.
Kim Y, Linkswiler HM (1979). Effect of level of protein intake on calcium metabolism and on parathyroid and renal function in the adult human male. J Nutr 109, 1399–1404.
Klahr S (1989). The modification of diet in renal disease study. N Engl J Med 320, 864–866.
Knight EL, Stampfer MJ, Hankinson SE, Spiegelman D, Curhan GC (2003). The impact of protein intake on renal function decline in women with normal renal function or mild renal insufficiency. Ann Int Med 138, 460–467.
Konturek SJ, Tasler J, Cieszkowski M, Jaworek J (1978). Comparison of intravenous amino acids in the stimulation of gastric secretion. Gastroenterology 75, 817–824.
Krieger NS, Sessler NE, Bushinsky DA (1992). Acidosis inhibits osteoblastic and stimulates osteoclastic activity in vitro. Am J Physiol Renal Physiol 262 (3 Pt 2), F442–F448.
Lacroix M, Gaudichon C, Martin A, Morens C, Mathe V, Tome D et al. (2004). A long-term high-protein diet markedly reduces adipose tissue without major side effects in Wistar male rats. Am J Physiol Regul Integr Comp Physiol 287, R934–R942.
Langdahl BL, Kassem M, Moller MK, Eriksen EF (1998). The effects of IGF-I and IGF-II on proliferation and differentiation of human osteoblasts and interactions with growth hormone. Eur J Clin Invest 28, 176–183.
Lei SF, Chen Y, Xiong DH, Li LM, Deng HW (2006). Ethnic difference in osteoporosis-related phenotypes and its potential underlying genetic determination. J Musculoskelet Neuronal Interact 6, 36–46.
Lemann Jr J, Gray RW, Pleuss JA (1989). Potassium bicarbonate, but not sodium bicarbonate, reduces urinary calcium excretion and improves calcium balance in healthy men. Kidney Int 35, 688–695.
Lemann Jr J, Pleuss JA, Gray RW (1993). Potassium causes calcium retention in healthy adults. J Nutr 123, 1623–1626.
Licata AA (1981). Acute effects of increased meat protein on urinary electrolytes and cyclic adenosine-monophosphate and serum parathyroid-hormone. Am J Clin Nutr 34, 1779–1784.
Lutz J (1984). Calcium balance and acid-base status of women as affected by increased protein intake and by sodium bicarbonate ingestion. Am J Clin Nutr 39, 281–288.
Lutz J, Linkswiler HM (1981). Calcium metabolism in postmenopausal and osteoporotic women consuming two levels of dietary protein. Am J Clin Nutr 34, 2178–2186.
Marangella M, Bagnis C, Bruno M, Vitale C, Petrarulo M, Ramello A (2004). Crystallization inhibitors in the pathophysiology and treatment of nephrolithiasis. Urol Int 72 (Suppl 1), 6–10.
Markovich D, Wang H, Puttaparthi K, Zajicek H, Rogers T, Murer H et al. (1999). Chronic K depletion inhibits renal brush border membrane Na/sulfate cotransport. Kidney Int 55, 244–251.
Martin WF, Armstrong LE, Rodriguez NR (2005). Dietary protein intake and renal function. Nutr Metab (Lond) 2, 25.
McCance RA, Widdowson EM, Lehmann H (1942). The effect of protein intake on the absorption of calcium and magnesium. Biochem J 36, 686–691.
Metges CC, Barth CA (2000). Metabolic consequences of a high dietary-protein intake in adulthood: assessment of the available evidence. J Nutr 130, 886–889.
Meyer HE, Pedersen JI, Loken EB, Tverdal A (1997). Dietary factors and the incidence of hip fracture in middle-aged Norwegians. A prospective study. Am J Epidemiol 145, 117–123.
Misra D, Berry SD, Broe KE, McLean RR, Cupples LA, Tucker KL et al. (2010). Does dietary protein reduce hip fracture risk in elders? The Framingham osteoporosis study. Osteoporos Int 22, 345–349.
Mohan S, Strong DD, Lempert UG, Tremollieres F, Wergedal JE, Baylink DJ (1992). Studies on regulation of insulin-like growth factor binding protein (IGFBP)-3 and IGFBP-4 production in human bone cells. Acta Endocrinol (Copenh) 127, 555–564.
Muhlbauer RC, Lozano A, Reinli A (2002). Onion and a mixture of vegetables, salads, and herbs affect bone resorption in the rat by a mechanism independent of their base excess. J Bone Miner Res 17, 1230–1236.
Munger RG, Cerhan JR, Chiu BC (1999). Prospective study of dietary protein intake and risk of hip fracture in postmenopausal women. Am J Clin Nutr 69, 147–152.
Nelson DA, Megyesi MS (2004). Sex and ethnic differences in bone architecture. Curr Osteoporos Rep 2, 65–69.
New SA (2002). Nutrition Society Medal lecture. The role of the skeleton in acid-base homeostasis. Proc Nutr Soc 61, 151–164.
New SA (2003). Intake of fruit and vegetables: implications for bone health. Proc Nutr Soc 62, 889–899.
New SA, Bolton-Smith C, Grubb DA, Reid DM (1997). Nutritional influences on bone mineral density: a cross-sectional study in premenopausal women. Am J Clin Nutr 65, 1831–1839.
New SA, Robins SP, Campbell MK, Martin JC, Garton MJ, Bolton-Smith C et al. (2000). Dietary influences on bone mass and bone metabolism: further evidence of a positive link between fruit and vegetable consumption and bone health? Am J Clin Nutr 71, 142–151.
Nguyen QV, Kalin A, Drouve U, Casez JP, Jaeger P (2001). Sensitivity to meat protein intake and hyperoxaluria in idiopathic calcium stone formers. Kidney Int 59, 2273–2281.
Pannemans DLE, Schaafsma G, Westerterp KR (1997). Calcium excretion, apparent calcium absorption and calcium balance in young and elderly subjects: influence of protein intake. Br J Nutr 77, 721–729.
Pedrini MT, Levey AS, Lau J, Chalmers TC, Wang PH (1996). The effect of dietary protein restriction on the progression of diabetic and nondiabetic renal diseases: a meta-analysis. Ann Int Med 124, 627–632.
Rafferty K, Davies KM, Heaney RP (2005). Potassium intake and the calcium economy. J Am Coll Nutr 24, 99–106.
Rafferty K, Heaney RP (2008). Nutrient effects on the calcium economy: emphasizing the potassium controversy. J Nutr 138, 166S–171S.
Reddy ST, Wang CY, Sakhaee K, Brinkley L, Pak CYC (2002). Effect of low-carbohydrate high-protein diets on acid-base balance, stone-forming propensity, and calcium metabolism. Am J Kidney Dis 40, 265–274.
Remer T (2000). Influence of diet on acid-base balance. Semin Dial 13, 221–226.
Remer T, Manz F (1994). Estimation of the renal net acid excretion by adults consuming diets containing variable amounts of protein. Am J Clin Nutr 59, 1356–1361.
Robertson JL, Goldschmidt M, Kronfeld DS, Tomaszewski JE, Hill GS, Bovee KC (1986). Long-term renal responses to high dietary protein in dogs with 75% nephrectomy. Kidney Int 29, 511–519.
Robertson L, Waugh N, Robertson A (2007). Protein restriction for diabetic renal disease. Cochrane Database Systematic Review. Article number: (4): CD002181.
Robertson WG, Heyburn PJ, Peacock M, Hanes FA, Swaminathan R (1979). The effect of high animal protein intake on the risk of calcium stone-formation in the urinary tract. Clin Sci (Lond) 57, 285–288.
Roughead ZK, Johnson LK, Lykken GI, Hunt JR (2003). Controlled high meat diets do not affect calcium retention or indices of bone status in healthy postmenopausal women. J Nutr 133, 1020–1026.
Rubin J, Ackert-Bicknell CL, Zhu L, Fan X, Murphy TC, Nanes MS et al. (2002). IGF-I regulates osteoprotegerin (OPG) and receptor activator of nuclear factor-kappa B ligand in vitro and OPG in vivo. J Clin Endocrinol Metab 87, 4273–4279.
Schuette SA, Linkswiler HM (1982). Effects on Ca and P metabolism in humans by adding meat, meat plus milk, or purified proteins plus Ca and P to a low protein diet. J Nutr 112, 338–349.
Schuette SA, Zemel MB, Linkswiler HM (1980). Studies on the mechanism of protein-induced hypercalciuria in older men and women. J Nutr 110, 305–315.
Schulte-Frohlinde E, Schmid R, Schusdziarra V (1993). Effect of a postprandial amino acid pattern on gastric acid secretion in man. Z Gastroenterol 31, 711–715.
Schurch MA, Rizzoli R, Slosman D, Vadas L, Vergnaud P, Bonjour JP (1998). Protein supplements increase serum insulin-like growth factor-I levels and attenuate proximal femur bone loss in patients with recent hip fracture. A randomized, double-blind, placebo-controlled trial. Ann Int Med 128, 801–809.
Sebastian A, Harris ST, Ottaway JH, Todd KM, Morris Jr RC (1994). Improved mineral balance and skeletal metabolism in postmenopausal women treated with potassium bicarbonate. N Engl J Med 330, 1776–1781.
Sellmeyer DE, Stone KL, Sebastian A, Cummings SR (2001). A high ratio of dietary animal to vegetable protein increases the rate of bone loss and the risk of fracture in postmenopausal women. Study of Osteoporotic Fractures Research Group. Am J Clin Nutr 73, 118–122.
Skov AR, Toubro S, Bulow J, Krabbe K, Parving HH, Astrup A (1999). Changes in renal function during weight loss induced by high vs low-protein low-fat diets in overweight subjects. Int J Obes Relat Metab Disord 23, 1170–1177.
Spencer H, Kramer L, DeBartolo M, Norris C, Osis D (1983). Further studies of the effect of a high protein diet as meat on calcium metabolism. Am J Clin Nutr 37, 924–929.
Spencer H, Kramer L, Osis D (1988). Do protein and phosphorus cause calcium loss? J Nutr 118, 657–660.
Spencer H, Kramer L, Osis D, Norris C (1978). Effect of a high-protein (meat) intake on calcium-metabolism in man. Am J Clin Nutr 31, 2167–2180.
Strunz UT, Walsh JH, Grossman MI (1978). Stimulation of gastrin release in dogs by individual amino acids. Proc Soc Exp Biol Med 157, 440–441.
Taylor EN, Stampfer MJ, Mount DB, Curhan GC (2010). DASH-style diet and 24-hour urine composition. J Am Soc Nephrol 5, 2315–2322.
Teegarden D, Lyle RM, McCabe GP, McCabe LD, Proulx WR, Michon K et al. (1998). Dietary calcium, protein, and phosphorus are related to bone mineral density and content in young women. Am J Clin Nutr 68, 749–754.
Thissen JP, Ketelslegers JM, Underwood LE (1994). Nutritional regulation of the insulin-like growth factors. Endocr Rev 15, 80–101.
Thorpe DL, Knutsen SF, Beeson WL, Rajaram S, Fraser GE (2008). Effects of meat consumption and vegetarian diet on risk of wrist fracture over 25 years in a cohort of peri- and postmenopausal women. Public Health Nutr 11, 564–572.
Tkatch L, Rapin CH, Rizzoli R, Slosman D, Nydegger V, Vasey H et al. (1992). Benefits of oral protein supplementation in elderly patients with fracture of the proximal femur. J Am Coll Nutr 11, 519–525.
Tosukhowong P, Tungsanga K, Phongudom S, Sriboonlue P (2005). Effects of potassium-magnesium citrate supplementation on cytosolic ATP citrate lyase and mitochondrial aconitase activity in leukocytes: a window on renal citrate metabolism. Int J Urol 12, 140–144.
Trilok G, Draper HH (1989). Sources of protein-induced endogenous acid production and excretion by human adults. Calcif Tissue Int 44, 335–338.
Trinchieri A, Lizzano R, Marchesotti F, Zanetti G (2006). Effect of potential renal acid load of foods on urinary citrate excretion in calcium renal stone formers. Urol Res 34, 1–7.
Tucker KL, Hannan MT, Chen H, Cupples LA, Wilson PW, Kiel DP (1999). Potassium, magnesium, and fruit and vegetable intakes are associated with greater bone mineral density in elderly men and women. Am J Clin Nutr 69, 727–736.
Tucker KL, Hannan MT, Kiel DP (2001). The acid-base hypothesis: diet and bone in the Framingham Osteoporosis Study. Eur J Nutr 40, 231–237.
Tuttle KR, Puhlman ME, Cooney SK, Short RA (2002). Effects of amino acids and glucagon on renal hemodynamics in type 1 diabetes. Am J Physiol Renal Physiol 282, F103–F112.
Wagner EA, Falciglia GA, Amlal H, Levin L, Soleimani M (2007). Short-term exposure to a high-protein diet differentially affects glomerular filtration rate but not acid-base balance in older compared to younger adults. J Am Diet Assoc 107, 1404–1408.
Walker RM, Linkswiler HM (1972). Calcium retention in the adult human male as affected by protein intake. J Nutr 102, 1297–1302.
Wasserstein AG, Stolley PD, Soper KA, Goldfarb S, Maislin G, Agus Z (1987). Case-control study of risk factors for idiopathic calcium nephrolithiasis. Miner Electrolyte Metab 13, 85–95.
Wengreen HJ, Munger RG, West NA, Cutler DR, Corcoran CD, Zhang J et al. (2004). Dietary protein intake and risk of osteoporotic hip fracture in elderly residents of Utah. J Bone Miner Res 19, 537–545.
Whiting SJ, Anderson DJ, Weeks SJ (1997). Calciuric effects of protein and potassium bicarbonate but not of sodium chloride or phosphate can be detected acutely in adult women and men. Am J Clin Nutr 65, 1465–1472.
Whiting SJ, Boyle JL, Thompson A, Mirwald RL, Faulkner RA (2002). Dietary protein, phosphorus and potassium are beneficial to bone mineral density in adult men consuming adequate dietary calcium. J Am Coll Nutr 21, 402–409.
Wilson HE (1933). An investigation of the cause of renal hypertrophy in rats fed on a high protein diet. Biochem J 27, 1348–1356.
Wolfe RR (2006). The underappreciated role of muscle in health and disease. Am J Clin Nutr 84, 475–482.
Zemel MB, Schuette SA, Hegsted M, Linkswiler HM (1981). Role of the sulfur-containing amino-acids in protein-induced hypercalciuria in men. J Nutr 111, 545–552.
Zerwekh JE, Odvina CV, Wuermser LA, Pak CYC (2007). Reduction of renal stone risk by potassium-magnesium citrate during 5 weeks of bed rest. J Urol 177, 2179–2184.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Rights and permissions
About this article
Cite this article
Calvez, J., Poupin, N., Chesneau, C. et al. Protein intake, calcium balance and health consequences. Eur J Clin Nutr 66, 281–295 (2012). https://doi.org/10.1038/ejcn.2011.196
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ejcn.2011.196