Abstract
Background/Objective:
The sweet-taste receptor (T1r2+T1r3) is expressed by enteroendocrine L-cells throughout the gastrointestinal tract. Application of sucralose (a non-calorific, non-metabolisable sweetener) to L-cells in vitro stimulates glucagon-like peptide (GLP)-1 secretion, an effect that is inhibited with co-administration of a T1r2+T1r3 inhibitor. We conducted a randomised, single-blinded, crossover study in eight healthy subjects to investigate whether oral ingestion of sucralose could stimulate L-cell-derived GLP-1 and peptide YY (PYY) release in vivo.
Methods:
Fasted subjects were studied on 4 study days in random order. Subjects consumed 50 ml of either water, sucralose (0.083% w/v), a non-sweet, glucose-polymer matched for sweetness with sucralose addition (50% w/v maltodextrin+0.083% sucralose) or a modified sham-feeding protocol (MSF=oral stimulation) of sucralose (0.083% w/v). Appetite ratings and plasma GLP-1, PYY, insulin and glucose were measured at regular time points for 120 min. At 120 min, energy intake at a buffet meal was measured.
Results:
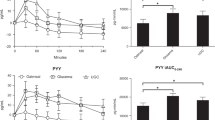
Sucralose ingestion did not increase plasma GLP-1 or PYY. MSF of sucralose did not elicit a cephalic phase response for insulin or GLP-1. Maltodextrin ingestion significantly increased insulin and glucose compared with water (P<0.001). Appetite ratings and energy intake were similar for all groups.
Conclusions:
At this dose, oral ingestion of sucralose does not increase plasma GLP-1 or PYY concentrations and hence, does not reduce appetite in healthy subjects. Oral stimulation with sucralose had no effect on GLP-1, insulin or appetite.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout

Similar content being viewed by others
References
Abdallah L, Chabert M, Louis-Sylvestre J (1997). Cephalic phase responses to sweet taste. Am J Clin Nutr 65, 737–743.
Adrian TE, Ferri GL, Bacarese-Hamilton AJ, Fuessl HS, Polak JM, Bloom SR (1985). Human distribution and release of a putative new gut hormone, peptide YY. Gastroenterology 89, 1070–1077.
Ahren B, Holst JJ (2001). The cephalic insulin response to meal ingestion in humans is dependent on both cholinergic and noncholinergic mechanisms and is important for postprandial glycemia. Diabetes 50, 1030–1038.
Batterham RL, Cowley MA, Small CJ, Herzog H, Cohen MA, Dakin CL et al. (2002). Gut hormone PYY(3-36) physiologically inhibits food intake. Nature 418, 650–654.
Bray GA, Nielsen SJ, Popkin BM (2004). Consumption of high-fructose corn syrup in beverages may play a role in the epidemic of obesity. Am J Clin Nutr 79, 537–543.
Brown RJ, Walter M, Rother KI (2009). Ingestion of diet soda before a glucose load augments GLP-1 secretion. Diabetes Care 32, 2184–2186.
Challis BG, Pinnock SB, Coll AP, Carter RN, Dickson SL, O’rahilly S (2003). Acute effects of PYY3-36 on food intake and hypothalamic neuropeptide expression in the mouse. Biochem Biophys Res Commun 311, 915–919.
Chelikani PK, Haver AC, Reeve Jr JR, Keire DA, Reidelberger RD (2006). Daily, intermittent intravenous infusion of peptide YY(3-36) reduces daily food intake and adiposity in rats. Am J Physiol Regul Integr Comp Physiol 290, R298–R305.
Chelikani PK, Haver AC, Reidelberger RD (2005a). Intravenous infusion of peptide YY(3-36) potently inhibits food intake in rats. Endocrinology 146, 879–888.
Chelikani PK, Haver AC, Reidelberger RD (2005b). Intravenous infusion of glucagon-like peptide-1 potently inhibits food intake, sham feeding, and gastric emptying in rats. Am J Physiol Regul Integr Comp Physiol 288, R1695–R1706.
Christofides ND, Sarson DL, Albuquerque RH, Adrian TE, Ghatei MA, Modlin IM et al. (1979). Release of gastrointestinal hormones following an oral water load. Experientia 35, 1521–1523.
Degen L, Oesch S, Casanova M, Graf S, Ketterer S, Drewe J et al. (2005). Effect of peptide YY3-36 on food intake in humans. Gastroenterology 129, 1430–1436.
Elliott SS, Keim NL, Stern JS, Teff K, Havel PJ (2002). Fructose, weight gain, and the insulin resistance syndrome. Am J Clin Nutr 76, 911–922.
Elrick H, Stimmler L, Hlad Jr CJ, Rai Y (1964). Plasma insulin response to oral and intravenous glucose administration. J Clin Endocrinol Metab 24, 1076–1082.
Flint A, Raben A, Astrup A, Holst JJ (1998). Glucagon-like peptide 1 promotes satiety and suppresses energy intake in humans. J Clin Invest 101, 515–520.
Flint A, Raben A, Blundell JE, Astrup A (2000). Reproducibility, power and validity of visual analogue scales in assessment of appetite sensations in single test meal studies. Int J Obes Relat Metab Disord 24, 38–48.
Fujita Y, Wideman RD, Speck M, Asadi A, King DS, Webber TD et al. (2009). Incretin release from gut is acutely enhanced by sugar but not by sweeteners in vivo. Am J Physiol Endocrinol Metab 296, E473–E479.
Ghatei MA, Uttenthal LO, Christofides ND, Bryant MG, Bloom SR (1983). Molecular forms of human enteroglucagon in tissue and plasma: plasma responses to nutrient stimuli in health and in disorders of the upper gastrointestinal tract. J Clin Endocrinol Metab 57, 488–495.
Grice HC, Goldsmith LA (2000). Sucralose—an overview of the toxicity data. Food Chem Toxicol 38 (Suppl 2), S1–S6.
Grotz VL, Henry RR, McGill JB, Prince MJ, Shamoon H, Trout JR et al. (2003). Lack of effect of sucralose on glucose homeostasis in subjects with type 2 diabetes. J Am Diet Assoc 103, 1607–1612.
Gutzwiller JP, Goke B, Drewe J, Hildebrand P, Ketterer S, Handschin D et al. (1999). Glucagon-like peptide-1: a potent regulator of food intake in humans. Gut 44, 81–86.
Halatchev IG, Ellacott KL, Fan W, Cone RD (2004). Peptide YY3-36 inhibits food intake in mice through a melanocortin-4 receptor-independent mechanism. Endocrinology 145, 2585–2590.
Hall WL, Millward DJ, Rogers PJ, Morgan LM (2003). Physiological mechanisms mediating aspartame-induced satiety. Physiol Behav 78, 557–562.
Jang HJ, Kokrashvili Z, Theodorakis MJ, Carlson OD, Kim BJ, Zhou J et al. (2007). Gut-expressed gustducin and taste receptors regulate secretion of glucagon-like peptide-1. Proc Natl Acad Sci USA 104, 15069–15074.
Kreymann B, Williams G, Ghatei MA, Bloom SR (1987). Glucagon-like peptide-1 7–36: a physiological incretin in man. Lancet 2, 1300–1304.
Le Roux CW, Batterham RL, Aylwin SJ, Patterson M, Borg CM, Wynne KJ et al. (2006). Attenuated peptide YY release in obese subjects is associated with reduced satiety. Endocrinology 147, 3–8.
Li X, Staszewski L, Xu H, Durick K, Zoller M, Adler E (2002). Human receptors for sweet and umami taste. Proc Natl Acad Sci USA 99, 4692–4696.
Luscombe-Marsh ND, Smeets AJ, Westerterp-Plantenga MS (2009). The addition of monosodium glutamate and inosine monophosphate-5 to high-protein meals: effects on satiety, and energy and macronutrient intakes. Br J Nutr 102, 929–937.
Ma J, Bellon M, Wishart JM, Young R, Blackshaw LA, Jones KL et al. (2009). Effect of the artificial sweetener, sucralose, on gastric emptying and incretin hormone release in healthy subjects. Am J Physiol Gastrointest Liver Physiol 296, G735–G739.
Morgan JF, Reid F, Lacey JH (2000). The SCOFF questionnaire: a new screening tool for eating disorders. West J Med 172, 164–165.
Murphy KG, Bloom SR (2006). Gut hormones and the regulation of energy homeostasis. Nature 444, 854–859.
Nelson G, Hoon MA, Chandrashekar J, Zhang Y, Ryba NJ, Zuker CS (2001). Mammalian sweet taste receptors. Cell 106, 381–390.
Parker HE, Reimann F, Gribble FM (2010). Molecular mechanisms underlying nutrient-stimulated incretin secretion. Expert Rev Mol Med 12, e1.
Raben A, Vasilaras TH, Moller AC, Astrup A (2002). Sucrose compared with artificial sweeteners: different effects on ad libitum food intake and body weight after 10 wk of supplementation in overweight subjects. Am J Clin Nutr 76, 721–729.
Reimann F, Habib AM, Tolhurst G, Parker HE, Rogers GJ, Gribble FM (2008). Glucose sensing in L cells: a primary cell study. Cell Metab 8, 532–539.
Rozengurt N, Wu SV, Chen MC, Huang C, Sternini C, Rozengurt E (2006). Colocalization of the alpha-subunit of gustducin with PYY and GLP-1 in L cells of human colon. Am J Physiol Gastrointest Liver Physiol 291, G792–G802.
Sutherland K, Young RL, Cooper NJ, Horowitz M, Blackshaw LA (2007). Phenotypic characterization of taste cells of the mouse small intestine. Am J Physiol Gastrointest Liver Physiol 292, G1420–G1428.
Talsania T, Anini Y, Siu S, Drucker DJ, Brubaker PL (2005). Peripheral exendin-4 and peptide YY(3-36) synergistically reduce food intake through different mechanisms in mice. Endocrinology 146, 3748–3756.
Teff KL, Devine J, Engelman K (1995). Sweet taste: effect on cephalic phase insulin release in men. Physiol Behav 57, 1089–1095.
Van Strien T, Rookus MA, Bergers GP, Frijters JE, Defares PB (1986). Life events, emotional eating and change in body mass index. Int J Obes 10, 29–35.
Wong GT, Gannon KS, Margolskee RF (1996). Transduction of bitter and sweet taste by gustducin. Nature 381, 796–800.
Acknowledgements
We thank Mandy Donaldson and John Meek for glucose and insulin assays, the Sir John McMichael research centre for Clinical Investigation and Research, Hammersmith Hospital and the volunteers. VP is funded through a European Union framework 6 Marie Curie fellowship (NuSISCO). NMM is funded by a HEFCE Clinical Senior Lecturer Award. This research is funded by program grants from the MRC (G7811974) and Wellcome Trust (072643/Z/03/Z) and by an EU FP6 Integrated Project Grant LSHM-CT-2003-503041. We are also grateful for support from the NIHR Biomedical Research Centre funding scheme. We thank Tate and Lyle for the provision of sucralose.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Contributors: HEF and VP designed the experiment, collected and analysed data and wrote the manuscript. NMM helped with the writing of the manuscript. MS contributed to the data analysis. MAG, GSF and SRB provided significant advice.
Rights and permissions
About this article
Cite this article
Ford, H., Peters, V., Martin, N. et al. Effects of oral ingestion of sucralose on gut hormone response and appetite in healthy normal-weight subjects. Eur J Clin Nutr 65, 508–513 (2011). https://doi.org/10.1038/ejcn.2010.291
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ejcn.2010.291
Keywords
This article is cited by
-
An alternative pathway for sweet sensation: possible mechanisms and physiological relevance
Pflügers Archiv - European Journal of Physiology (2020)
-
Effects of Non-nutritive Sweeteners on Sweet Taste Processing and Neuroendocrine Regulation of Eating Behavior
Current Nutrition Reports (2020)
-
Non-nutritive Sweeteners and Glycaemic Control
Current Atherosclerosis Reports (2019)
-
A workshop on ‘Dietary Sweetness—Is It an Issue?’
International Journal of Obesity (2018)
-
Unexpected behaviors in molecular transport through size-controlled nanochannels down to the ultra-nanoscale
Nature Communications (2018)