Abstract
Background/Objectives:
Protein concentration is lower in human milk (HM) than in infant formula. The objective of this study was to evaluate the effect of an α-lactalbumin-enriched formula with a lower protein concentration on infant growth, protein markers and biochemistries.
Subjects/Methods:
Healthy term formula-fed (FF) infants 5–14 days old were randomized in this controlled, double-blind trial to standard formula (SF: 14.1 g/l protein, 662 kcal/l) group (n=112) or experimental formula (EF: 12.8 g/l protein, 662 kcal/l) group (n=112) for 120 days; a HM reference group (n=112) was included. Primary outcome was weight gain (g/day) from D0 to D120. Secondary outcomes included serum albumin, plasma amino acids insulin and incidence of study events. Anthropometric measures were expressed as Z-scores using 2006 World Health Organization growth standards.
Results:
A total of 321 of the 336 infants (96%) who enrolled, completed the study. Mean age was 9.6 (±2.9) days; 50% were girls. Mean weight gain (g/day) did not significantly differ between SF vs EF (P=0.67) nor between EF vs HM (P=0.11); however weight gain (g/day) was significantly greater in the SF vs HM group (P=0.04). At day 120, mean weight-for-age Z-score (WAZ) and weight-for-length Z-score (WLZ) did not significantly differ between SF vs EF nor EF vs HM; however the WAZ was significantly greater in SF vs HM (P=0.025). Secondary outcomes were within normal ranges for all groups. Incidence of study events did not differ among groups.
Conclusions:
α-Lactalbumin-enriched formula containing12.8 g/l protein was safe and supported age-appropriate growth; weight gain with EF was intermediate between SF and HM groups and resulted in growth similar to HM-fed infants in terms of weight gain, WAZ and WLZ.
Similar content being viewed by others
Introduction
Human milk (HM) is the gold standard of early infant nutrition. The World Health Organization (World Health Organization, 2003) and the American Academy of Pediatrics (Koosha et al., 2008; CON AAP, 2009) recommend that infants be exclusively breastfed for the first 6 months of life. However, this recommendation is not consistently achieved; reported rates of exclusive breast feeding at 6 months of age range from 10 to 46% (World Health Organization, 2003; Koosha et al., 2008). The World Health Organization and AAP recognize infant formula as a suitable feeding alternative when HM is not available (World Health Organization, 2003; CON AAP, 2009). Mixed feeding (breastfed and infant formula) does occur, and it is estimated that between 35 and 50% of the infants receive some feedings of infant formula during the first 6 months of life (Bolling et al., 2007; Shealy et al., 2008). Given the number of infants receiving mixed feedings (HM and formula), and the importance of early life nutrition for optimal growth and development, continued research related to the composition of infant formula and health outcomes of formula fed (FF) infants is warranted.
One of the areas in which HM and infant formula differ is in protein composition and concentration. Mature HM provides 10–12 gm/l total protein (Lonnerdal et al., 1976; Raiha et al., 1986; Darragh and Moughan, 1998; Feng et al., 2009) and is rich in essential amino acids. Standard infant formulas are typically made from cow's milk, which has a higher total protein concentration and contains a different amino acid profile than HM. A higher total protein concentration in infant formula (14–15 gm/l) has been necessary to provide sufficient quantities of all the essential amino acids.
Higher protein concentration in infant formula is hypothesized to be a factor that contributes to growth differences observed between FF- and HM-fed infants (Koletzko et al., 2009). FF infants tend to have higher post prandial (Tikanoja and Simell, 1983) and fasting (Picone et al., 1989; Hanning et al., 1992; Akeson et al., 1998) concentrations of branched chain amino acids compared with HM-fed infants. Plasma concentrations of these amino acids have been positively correlated with insulin release (Ginsburg et al., 1984), and insulin is known to increase cellular glucose uptake and inhibit lypolysis. These metabolic alterations are proposed mechanisms for greater weight gain and greater weight-for-length z-scores (WLZ) or body mass index in FF infants compared with HM-fed infants (Lucas et al., 1980). Rapid weight gain, upward crossing of growth percentiles and a greater weight-for-length at 6 months have been identified as risk factors for overweight and obesity, later in life (Stettler et al., 2002, 2003; Karaolis-Danckert et al., 2007; Taveras et al., 2009).
The primary limiting factor in reducing the total protein concentration in infant formula is the ability to provide sufficient quantities of essential amino acids. This can, in part, be addressed by enriching whey protein fractions in formula with substantially higher concentrations of α-lactalbumin; α-lactalbumin is the predominant whey protein found in HM and is a rich source of essential amino acids (Lien et al., 2004). α-Lactalbumin accounts for 28% of the total protein in HM and only 3% of the total protein in bovine milk (Heine et al., 1991). Human and bovine α-lactalbumin share 72% amino acid sequence homology and both consist of 123 amino acids (Findlay and Brew, 1972). Infants fed a formula enriched with bovine α-lactalbumin (14 g/l protein, 2.2 g/l α-lactalbumin) demonstrated appropriate growth and biomarkers of protein status (Lien et al., 2004), as well as good gastrointestinal tolerance to the formula (Davis et al., 2008). It was notable that the plasma amino acid concentrations in the infants consuming the 14 g/l protein formula were similar to but slightly higher than those fed HM (Davis et al., 2008). These data suggested that a further reduction in protein concentration of an α-lactalbumin-enriched formula might be achievable, while still supporting appropriate growth.
This study sought to evaluate the safety and efficacy of a new, lower protein, α-lactalbumin-enriched formula. The primary hypothesis was that weight gain of infants fed this lower protein experimental formula (EF) would be less than but within 3 g/day of those fed the higher protein standard formula (SF), which in addition to other parameters, would indicate that the lower protein concentration was indeed suitable for term infants. We also sought to evaluate whether the modest reduction in the formula's total protein concentration would result in growth outcomes in EF infants that were similar to HM-fed infants.
Subjects and methods
Study design
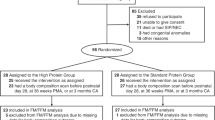
This study was a randomized, controlled, double-blind study of healthy term FF infants; FF infants were randomized to receive either SF or EF, and a HM group was included as a reference. The protocol and informed consent form were reviewed and approved by the National Ethics Committee and the Bureau of Food and Drugs in the Philippines. Written informed consent was obtained from the parent or legal guardian of each infant before enrollment.
Subjects
Inclusion criteria required that infants were at birth, a singleton and term infant; at enrollment were 5–14 days of age, and had a weight, length and head circumference ⩾fifth and ⩽ninety-fifth percentile for age according to Filipino growth reference standards (Florentino et al., 1992), and exclusively consuming and tolerating a cow's milk infant formula (to be eligible for FF group), or exclusively consuming and tolerating HM (to be eligible for HM group). Main exclusion criteria included: a family history of allergy to a cow's milk protein formula, major congenital malformations, infection or other systemic disorders.
Methods
Randomization
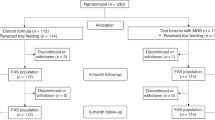
Allocation of FF infants to one of the two study formulas proceeded through the use of a gender-stratified randomization schedule. To ensure double blinding, the packaging of the study formula was identical aside from the package number. Formula feeding began after randomization (baseline) and continued for 120 days.
Nutrient composition of study formulas
Study formulas, standard S-26 GOLD (SF) or experimental S-26 GOLD (EF) (Wyeth Nutrition, Askeaton, Ireland) were packaged in 250 ml ready-to-feed Tetra Brik cartons (Tetra Pak, Lund, Sweden). The main difference between the formulas (Table 1) was the total protein concentration. The ability to provide the required amount of essential amino acids in a formula with a lower protein concentration was achieved primarily through enrichment of the formula with α-lactalbumin and supplementation with small amounts of L-tyrosine and L-tryptophan. The vitamin and mineral concentrations of the study formulas were similar. Both formulas satisfied the essential composition requirements of Codex and EU Commissions (Codex Alimentarius Commission, 1981; European Commission, 2006) and the US Infant Formula Act (United States Food and Drug Administration, 2004).
Anthropometric measures
Weight, recumbent length and head circumference were measured at baseline, days 30, 60, 90 and 120. Weight of naked infants was measured on an infant scale (Seca 374, Hamburg, Germany), recumbent length was measured on a pediatric length board (Ellard Instrumentation, Washington, DC, USA) and head circumference was measured utilizing a pediatric tape measure (Seca 212, Hamburg, Germany). All anthropometric measures were taken twice at each visit and the mean was calculated.
Serum/plasma biochemistries
Blood samples were collected at baseline, study days 60 and 120. At day 60, a timed blood sample (2 h post-prandial) was collected to assess plasma essential and conditionally essential amino acids, insulin and glucose concentrations. Serum chemistries were analyzed by spectrophotometry, serum insulin was measured by radioimmunoassay and serum glucose was measured by spectrophotometry (Quest Diagnostics Clinical Trials, Valencia, CA, USA). Plasma amino acids were analyzed by ion-exchange chromatography with the exception of tryptophan, which was analyzed by tandem liquid chromatography mass spectroscopy (Mayo Clinical Trial Services, Rochester, MN, USA).
Study events
Study events were recorded throughout the study. A study event was defined as any untoward, undesired or unplanned event in the form of signs, symptoms, disease or laboratory or physiological observations occurring in a person given a test article (study formula) or enrolled in the study. The investigator assigned each event as ‘related’ if there was a reasonable causal relationship to the test article, or ‘not related’ if there was not a reasonable causal relationship to the test article. A subset of symptoms related to the digestive system and gastrointestinal tolerance were identified of particular interest a priori: hard stool, constipation, difficulty having a bowel movement, acute diarrhea, chronic diarrhea, spitting up, regurgitation, vomiting, gastroesophageal reflux disease, colic and crying/neonatal abnormal crying. To ensure consistency in diagnosis, investigators were provided with standard definitions for these symptoms.
Data management
Data management was performed by Accenture (Philadelphia, PA, USA). Remote data capture was used to electronically record data; study monitors verified data in RDC against the source data and identified and resolved discrepancies. Data collection, data entry, query process, data review and database lock were performed according to standard operating procedures.
Statistical analysis
Data analysis was conducted as outlined in the statistical analysis plan utilizing SAS software version 9.1.3 (Cary, NC, USA). Analysis populations were defined as follows, intent to treat: subjects with a randomization number and efficacy analyzable: all randomized infants who took at least one feed of the study formula and have a measurable primary endpoint. Anthropometric measures were evaluated as raw data and also expressed as z-scores relative to the growth standards of the World Health Organization (WHO Multicentre Growth Reference 2006) using a World Health Organization software program (http://www.who.int/childgrowth/software/en/). Baseline z-score values are raw means; study day z-score values are adjusted means based on an ANCOVA with baseline and feeding group in the model.
Comparison between the EF and SF groups was of interest, as was the comparisons between each formula group and the HM group. Analyses were conducted as independent sets of pairwise comparisons because the HM group was included as a reference group and was not a randomized group. A sample size of 90 subjects per arm (45 subjects per gender per arm) was required to have 80% power to detect a difference in weight gain of 3 g/day (baseline to day 120) between groups, when testing at an α level of 0.025 (one-tailed). The error rate was held constant at the α=0.05 level for all outcomes. Given the multiple t-tests performed for the plasma essential amino acid analysis, results are also present using the Bonferroni adjustment for multiplicity αadj=0.0015.
Results
Subject disposition, study events and subject demography
Of the 336 infants enrolled (112 infants per group), 321 infants completed the study (96%). The number of discontinuations for study events was low and not significantly different between groups (2.7, 2.7 and 0% in the SF, EF and HM groups, respectively). The primary safety endpoint was the frequency of study events; study events were distributed across all treatment groups. A total of 25 treatment-related gastrointestinal (GI) study events occurred (see Methods): 6.3% in the EF group, 11.6% in the SF group and 4.5% in the HM group. Baseline infant characteristics were comparable between the groups (Table 2), with the exception of birth weight, which was significantly higher in the formula groups compared with HM (P=0.005, HM vs EF or SF). Mothers of FF infants gained significantly (P=0.014) more weight during pregnancy compared with mothers of HM-fed infants (EF: 11.8 kg, SF: 11.2 kg and HM: 10.2 kg).
Growth velocity
Mean weight gain (baseline to day 120 g/day) did not significantly differ between neither EF vs SF (P=0.67) nor EF vs HM (P=0.11); however, weight gain was significantly greater in the SF vs HM group (P=0.04) (Table 3). Mean length gain (baseline to day 120) was comparable and did not significantly differ between EF vs SF. Mean length gain of the EF group was slightly greater than that of the HM infants (P=0.03).
Z-scores
At baseline, there were no significant differences between the EF and SF groups in weight-for-age z-score (WAZ), length-for-age z-score, head circumference-for-age z-score or WLZ (Figure 1). Both formula groups were significantly higher than HM group at baseline for WAZ (P=0.008 and P=0.012 for EF and SF, respectively) and head circumference-for-age z-score (P=0.03 and P=0.003 for EF and SF, respectively). The mean WLZ of SF group was significantly higher than the HM group (P=0.03) at baseline.
Z-scores are based on the World Health Organization reference data (World Health Organization, 2003). (a) weight-for-age Z-score, (b) length-for-age Z-score, (c) head circumference-for age-Z-score, (d) weight-for-length Z-score. Baseline values are means; P-values are based on a two-sample t-test. Study day values are least square (LS) means (study day mean adjusted for baseline); P-value is based on ANCOVA with baseline value and feeding group in the model. Significance declared at P<0.05; **Significant difference between SF and HM groups; *Significant difference between EF and HM groups.
At day 120, there were no significant differences between the EF and SF groups for any mean z-score parameters (adjusted for baseline). The adjusted mean WAZ of the SF group was significantly higher than the HM group (mean difference 0.245, P=0.03). The adjusted mean length-for-age z-score of the EF and SF groups were significantly higher than the HM group (mean difference 0.269 and 0.243, P=0.001 and P=0.002, for EF and SF groups, respectively). The adjusted mean head circumference-for-age z-score of the EF and SF groups were significantly higher than HM (mean difference 0.227 and 0.219, P=0.007 and P=0.009 for EF and SF groups, respectively). There was no significant difference in the adjusted mean WLZ for either formula group against HM at study day 120.
Serum biochemistries and amino acids
Mean concentrations of serum albumin, total protein, blood urea nitrogen and creatinine were within the normal range for all groups at all time points (Table 4). Mean concentrations of glucose and insulin did not differ between groups. There were no significant differences in the mean amino acid concentrations between the EF and SF groups (Table 5). EF and SF infants had a significantly greater concentrations of some amino acids (lysine, methionine, phenylalanine and threonine) compared with the HM group, however, the mean concentrations of these amino acids were within the normal range for all groups. In general, the plasma essential amino acid concentrations of both the EF and SF formula groups were similar to and within one s.d. of the HM group.
Discussion
This study demonstrated that healthy term infants fed a new lower protein formula (EF) had an age-appropriate growth, biomarkers of protein status and biochemistries were normal, and no difference in the incidence of study events compared with the SF and HM groups. Weight gain with the new lower protein formula (EF) was intermediate between SF and HM groups; infants who consumed the new formula had weight gain, WAZ and WLZ similar to the HM-fed infant. This study compared growth of infants fed formulas that had a very modest difference (∼10%) in protein concentration; both formulas were found to be safe and support growth, but notably the weight gain in the lower protein (EF) group was not significantly different from the HM group.
Evidence of a positive relationship between the protein concentration of feeding and weight gain is hypothesized (Koletzko et al., 2009), and supported by the results of randomized and observational studies. In prospective randomized trials, infants receiving a higher protein formula have been shown to have greater weight gain (Raiha et al., 1986; Axelsson et al., 1989) or greater WAZ (Koletzko et al., 2009) compared with infants receiving a lower protein formula. Randomized trials that have not found this difference (Janas et al., 1987; Picone et al., 1989; Turck et al., 2006) tended to have a smaller difference in protein concentration between the formulas as well as a smaller sample size. In observational cohort studies, a higher protein intake (Gunnarsdottir and Thorsdottir, 2003) and a higher percentage of dietary energy as protein (Rolland-Cachera et al., 1995; Scaglioni et al., 2000) have been associated with overweight or increased body mass index at an age of 5–8 years, and an association between weight gain in infancy and later life obesity has also been shown (Stettler et al., 2002; Ong et al., 2009). In summary, the literature suggests that the protein concentration of infant feeding has a role in weight gain during infancy, which in turn may have effect on weight in later life; this underscores the importance of the results of weight gain observed in the lower protein (EF) group in this study.
The difference in weight gain between the EF and SF groups was 0.3 g/day and although this represents only a modest reduction on a daily basis, it is cumulative and may be meaningful when projected over a longer time period. Indeed, at the conclusion of the study the mean adjusted WAZ of infants fed EF was not significantly different from that of HM group, whereas those receiving the SF had significantly greater WAZ compared with that of HM group. Both the formula groups had a significantly greater rate of length gain and length-for-age Z-scores compared with that of the HM group. Although the formula groups were longer than their HM-fed counterparts, their weight was proportional to their length as demonstrated by no significant difference in the WLZ of either of the formula group against the HM group at the conclusion of the study.
The plasma essential and conditional amino acid concentrations of infants fed with EF were for the most part intermediate between the SF and HM groups. Higher concentrations of the branched chain amino acids leucine, isoleucine and valine have been positively correlated with insulin concentrations (Ginsburg et al., 1984), which in turn may affect weight gain. This study found no significant difference in insulin concentrations between any of the groups. Higher insulin concentration in FF infants compared with HM-fed infants found in an earlier study (Lucas et al., 1980) may be because of substantially higher total protein concentration of infant formulas at that time.
The strengths of this study include the sample size, gender stratification and completion rate. The completion rate exceeded the minimum sample size needed to test the primary hypothesis, and gender balance was achieved within each group. A limitation of the study relates to the duration of the clinical trial infants received the study formula until they were 4.5 months of age. We do not know whether the weight gain observed in the EF group will have an impact on weight status in later age.
In summary, a randomized clinical trial demonstrated that the new lower protein α-lactalbumin-enriched formula (EF) is appropriate for term infants as evidenced by age-appropriate growth, markers of protein status, plasma essential amino acid concentrations and gastrointestinal tolerance. Infants randomized to the lower protein formula had growth outcomes similar to HM-fed infants in terms of weight gain, WAZ and WLZ. These data suggest that a modest reduction in the total protein concentration of the formula has an effect on growth in the short term; further studies are needed to determine if the trend in weight gain observed in the EF group has an impact on weight status in later life.
References
Akeson PM, Axelsson IE, Raiha NC (1998). Protein and amino acid metabolism in three- to twelve-month-old infants fed human milk or formulas with varying protein concentrations. J Pediatr Gastroenterol Nutr 26, 297–304.
Axelsson IE, Ivarsson SA, Raiha NC (1989). Protein intake in early infancy: effects on plasma amino acid concentrations, insulin metabolism, and growth. Pediatr Res 26, 614–617.
Bolling K, Grant C, Hamlyn B, Thornton A (2007). The use of milk other than breast milk. Infant Feeding Survey 2005. Information Centre: London, England. pp 169–204.
Codex Alimentarius Commission (1981). Standard for Infant Formula and Formulas for Special Medical Purposes Intended for Infants CODEX STAN 72 – 1981.
Committee on Nutrition, American Academy of Pediatrics (2009). Formula feeding of term infants. In: Kleinman RE (ed). Pediatric Nutrition Handbook, 6th edn. American Academy of Pediatrics: Elk Grove Village, IL, pp 61–78.
Darragh AJ, Moughan PJ (1998). The amino acid composition of human milk corrected for amino acid digestibility. Br J Nutr 80, 25–34.
Davis AM, Harris BJ, Lien EL, Pramuk K, Trabulsi J (2008). [Alpha]-lactalbumin-rich infant formula fed to healthy term infants in a multicenter study: plasma essential amino acids and gastrointestinal tolerance. Eur J Clin Nut r 62, 1294–1301.
European Commission (2006). Commission Directive 2006/141/EC of 22 December 2006 on infant formulae and follow-on formulae and amending Directive 1999/21/EC Text with EEA relevance.
Feng P, Gao M, Holley T, Zhou T, Burgher A, Trabulsi J et al. (2009). Amino acid composition and protein content of mature human milk from nine countries. FASEB J 23, LB448.
Findlay JBC, Brew K (1972). The complete amino-acid sequence of human alpha-lactalbumin. Eur J Biochem 27, 65–86.
Florentino RF, Santos-Ocampo PD, Magbitang JA, Mendoza TS, Flores EG, Madrid BJ (1992). FNRI-PPA Anthropometric Tables and Charts for Filipino Children. Food and Nutrition Research Institute and Philippine Pediatric Society: Manila, Philippines.
Ginsburg BE, Lindblad BS, Lundsjo A, Persson B, Zetterstrom R (1984). Plasma valine and urinary C-peptide in breast-fed and artificially fed infants up to 6 months of age. Acta Paediatr 73, 213–217.
Gunnarsdottir I, Thorsdottir I (2003). Relationship between growth and feeding in infancy and body mass index at the age of 6 years. Int J Obes 27, 1523–1527.
Hanning RM, Paes B, Atkinson SA (1992). Protein metabolism and growth of term infants in response to a reduced- protein, 40:60 whey: casein formula with added tryptophan. Am J Clin Nutr 56, 1004–1011.
Heine WE, Klein PD, Reeds PJ (1991). The Importance of [alpha]-Lactalbumin in Infant Nutrition. J Nutr 121; 277–283.
Janas LM, Picciano MF, Hatch TF (1987). Indices of protein metabolism in term infants fed either human milk or formulas with reduced protein concentration and various whey/casein ratios. J Pediatr 110, 838–848.
Karaolis-Danckert N, Gunther ALB, Kroke A, Hornberg C, Buyken AE (2007). How early dietary factors modify the effect of rapid weight gain in infancy on subsequent body-composition development in term children whose birth weight was appropriate for gestational age. Am J Clin Nutr 86, 1700–1708.
Koletzko B, von Kries R, Closa R, Escribano J, Scaglioni S, Giovannini M et al. (2009). Lower protein in infant formula is associated with lower weight up to age 2 y: a randomized clinical trial. Am J Clin Nutr 89, 1836–1845.
Koosha A, Hashemifesharaki R, Mousavinasab N (2008). Breast-feeding patterns and factors determining exclusive breast-feeding. Singapore Med J 49, 1002–1006.
Lien EL, Davis AM, Euler AR, Multicenter Study Group (2004). Growth and safety in term infants fed reduced-protein formula with added bovine alpha-lactalbumin. J Pediatr Gastroenterol Nutr 38, 170–176.
Lonnerdal B, Forsum E, Gebre-Medhin M, Hambraeus L (1976). Breast milk composition in Ethiopian and Swedish mothers. II. Lactose, nitrogen, and protein contents. Am J Clin Nutr 29, 1134–1141.
Lucas A, Blackburn AM, Aynsley-Green A, Sarson DL, Adrian TE, Bloom SR (1980). Breast vs bottle: endocrine responses are different with formula feeding. Lancet 315, 1267–1269.
Ong KK, Langkamp M, Ranke MB, Whitehead K, Hughes IA, Acerini CL et al. (2009). Insulin-like growth factor I concentrations in infancy predict differential gains in body length and adiposity: the Cambridge Baby Growth Study. Am J Clin Nutr 90, 156–161.
Picone TA, Benson JD, Moro G, Minoli I, Fulconis F, Rassin DK et al. (1989). Growth, serum biochemistries, and amino acids of term infants fed formulas with amino acid and protein concentrations similar to human milk. J Pediatr Gastroenterol Nutr 9, 351–360.
Raiha N, Minoli I, Moro G (1986). Milk protein intake in the term infant. I. Metabolic responses and effects on growth. Acta Paediatr 75, 881–886.
Rolland-Cachera MF, Deheeger M, Akrout M, Bellisle F (1995). Influence of macronutrients on adiposity development: a follow up study of nutrition and growth from 10 months to 8 years of age. Int J Obes Relat Metab Disord 19, 573–578.
Scaglioni S, Agostoni C, De Notaris R, Radaelli G, Radice N, Valenti M et al. (2000). Early macronutrient intake and overweight at five years of age. Int J Obes Relat Metab Disord 24, 777–781.
Shealy KR, Scanlon KS, Labiner-Wolfe J, Fein SB, Grummer-Strawn LM (2008). Characteristics of breastfeeding practices among US mothers. Pediatrics 122, S50–S55.
Stettler N, Kumanyika SK, Katz SH, Zemel BS, Stallings VA (2003). Rapid weight gain during infancy and obesity in young adulthood in a cohort of African Americans. Am J Clin Nutr 77, 1374–1378.
Stettler N, Zemel BS, Kumanyika S, Stallings VA (2002). Infant weight gain and childhood overweight status in a multicenter, cohort study. Pediatrics 109, 194–199.
Taveras EM, Rifas-Shiman SL, Belfort MB, Kleinman KP, Oken E, Gillman MW (2009). Weight status in the first 6 months of life and obesity at 3 years of age. Pediatrics 123, 1177–1183.
Tikanoja T, Simell O (1983). Plasma amino acids after a feed of human milk or formula at three months of age. J Pediatr Gastroenterol Nutr 2, 252–255.
Turck D, Grillon C, Lachambre E, Robiliard P, Beck L, Maurin JL et al. (2006). Adequacy and safety of an infant formula with a protein/energy ratio of 1.8 g/100 kcal and enhanced protein efficiency for term infants during the first 4 months of life. J Pediatr Gastroenterol Nutr 43, 364–371.
United States Food and Drug Administration (2004). Federal Food, Drug and Cosmetic Act; Section 412. (21 USC §350a) Requirements for Infant Formulas. http://www.fda.gov/RegulatoryInformation/Legislation/FederalFoodDrugandCosmeticActFDCAct/FDCActChapterIVFood/ucm107864.htm.
World Health Organization (2003). Global Strategy for Infant and Young Children Feeding. World Health Organization: Geneva, Switzerland, 30pp.
Acknowledgements
This study was supported by Pfizer Nutrition.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
This research was supported by Wyeth Nutrition, Ireland. Dr Trabulsi, Dr Ramanujam, Dr McSweeney, Dr Harris, Dr DeRusso, and Ms Lebumfacil and Feng are employed by Wyeth Nutrition.
Rights and permissions
This work is licensed under the Creative Commons Attribution-NonCommercial-No Derivative Works 3.0 Unported License. To view a copy of this license, visit http://creativecommons.org/licenses/by-nc-nd/3.0/
About this article
Cite this article
Trabulsi, J., Capeding, R., Lebumfacil, J. et al. Effect of an α-lactalbumin-enriched infant formula with lower protein on growth. Eur J Clin Nutr 65, 167–174 (2011). https://doi.org/10.1038/ejcn.2010.236
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ejcn.2010.236
Keywords
This article is cited by
-
Cellular agriculture for milk bioactive production
Nature Reviews Bioengineering (2023)
-
Separation of Whey Protein Aggregates by Means of Continuous Centrifugation
Food and Bioprocess Technology (2019)
-
Health-related quality of life, temperament, and eating behavior among formula-fed infants in the Philippines: a pilot study
Health and Quality of Life Outcomes (2018)
-
Effects of cow milk versus extensive protein hydrolysate formulas on infant cognitive development
Amino Acids (2016)
-
Milk Feeding, Solid Feeding, and Obesity Risk: A Review of the Relationships Between Early Life Feeding Practices and Later Adiposity
Current Obesity Reports (2013)