Abstract
Background:
Dietary flaxseed may have beneficial cardiovascular effects. An aged population has a higher incidence of cardiovascular disease, but they may react differently to flaxseed in the diet.
Objective:
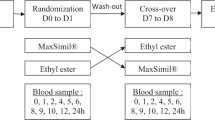
To investigate the response, over a period of 4 weeks, of subjects aged 18–29 or 45–69 years to a diet containing the same amount of α-linolenic acid (ALA) (6 g) introduced in the form of ground flaxseed (30 g) or flaxseed oil.
Results:
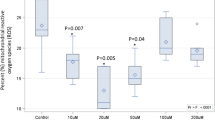
All subjects who received flaxseed oil showed a significant increase in plasma ALA and eicosapentaenoic acid (EPA) concentrations over the course of this study. Subjects who received ground flaxseed in the 18–29-year-old group showed a statistically significant increase in their plasma ALA levels, and although there was a trend in the same direction for the 45–69-year-old subjects, this did not achieve statistical significance. The diets induced no major changes in platelet aggregation, plasma total cholesterol, low-density lipoprotein or high-density lipoprotein cholesterol levels in any of the groups. Younger subjects showed a decrease in triglyceride (TG) values compared with older subjects. There were no significant side effects that caused compliancy issues.
Conclusion:
Subject age does not seem to be a major determining factor in influencing ALA absorption from a flaxseed-supplemented diet nor in the metabolism of ALA to EPA in the groups fed flaxseed oil. Concerns about side effects in older subjects administered a higher fiber load in a flaxseed-supplemented diet are not justified. However, younger but not older subjects showed a beneficial decrease in circulating TGs due to flaxseed supplementation.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout

Similar content being viewed by others
References
Akabas SR, Deckelbaum RJ (2006). Summary of a workshop on n-3 fatty acids: current status of recommendations and future directions. Am J Clin Nutr 83 (suppl), 1536S–1538S.
Allman MA, Pena NM, Pang D (1995). Supplementation with flaxseed oil versus sunflower oil in healthy young men consuming a low fat diet: effects on platelet composition and function. Eur J Clin Nutr 49, 169–178.
Ander BP, Weber AR, Rampersad PP, Gilchrist JS, Pierce GN, Lukas A (2004). Dietary flaxseed protects against ventricular fibrillation induced by ischemia-reperfusion in normal and hypercholesterolemic rabbits. J Nutr 134, 3250–3256.
Arjmandi BH, Khan DA, Juma S, Drum ML, Venkatesh S, Sohn E et al. (1998). Whole flaxseed consumption lowers serum LDL-cholesterol and lipoprotein(a) concentrations in postmenopausal women. Nutr Res 18, 203–1214.
Arterburn LM, Hall EB, Oken H (2006). Distribution, interconversion, and dose response of n-3 fatty acids in humans. Am J Clin Nutr 83 (suppl), 1467S–1476S.
Austria JA, Richard MN, Chahine MN, Edel AL, Malcolmson LJ, Dupasquier CMC et al. (2008). Bioavailability of alpha-linolenic acid in subjects after ingestion of three different forms of flaxseed. J Am Coll Nutr. 27, 214–221.
Cardinal DC, Flower RJ (1980). The electronic aggregometer: a novel device for assessing platelet behavior in blood. J Pharmacol Methods 3, 135–158.
Clark WF, Kortas C, Heidenheim AP, Garland J, Spanner E, Parbtani A (2001). Flaxseed in lupus nephritis: a two-year nonplacebo-controlled crossover study. J Am Coll Nutr 20, 143–148.
Clark WF, Parbtani A, Huff MW, Spanner E, De Saus H, Chin-Yee I et al. (1995). Flaxseed: a potential treatment for lupus nephritis. Kidney Int 48, 475–480.
Cunnane SC, Hamadeh MJ, Liede AC, Thompson LU, Wolever TM, Jenkins DJ (1995). Nutritional attributes of traditional flaxseed in healthy young adults. Am J Clin Nutr 61, 62–68.
de Lorgeril M, Renaud S, Mamelle N, Salen P, Martin JL, Monjaud I et al. (1994). Mediterranean á-linolenic acid-rich diet in secondary prevention of coronary heart disease. Lancet 343, 1454–1459.
de Lorgeril M, Salen P, Martin JL, Monjaud I, Delaye J, Mamelle N (1999). Mediterranean diet, traditional risk factors, and the rate of cardiovascular complications after myocardial infarction: final report of the Lyon Diet Heart Study. Circulation 99, 779–785.
Djoussé L, Hunt SC, Arnett DK, Province MA, Eckfeldt JH, Ellison RC (2003). Dietary linolenic acid is inversely associated with plasma triacylglycerol: the NHLBI Family Heart Study. Am J Clin Nutr 78, 1098–1102.
Dupasquier CMC, Dibrov E, Kneesh AL, Cheung PKM, Lee KGY, Alexander HK et al. (2007). Dietary flaxseed inhibits atherosclerosis in the LDL receptor deficient mouse in part through anti-proliferative and anti-inflammatory actions. Am J Physiol 293, H2394–H2402.
Dupasquier CM, Weber AM, Ander BP, Rampersad PP, Steigerwald S, Wigle JT et al. (2006). Effects of dietary flaxseed on vascular contractile function and atherosclerosis during prolonged hypercholesterolemia in rabbits. Am J Physiol Heart Circ Physiol 291, H2987–H2996.
Erkkilä AT, Lehto S, Pyörälä K, Uusitupa MIJ (2003). n-3 fatty acids and 5-y risks of death and cardiovascular disease events in patients with coronary artery disease. J Clin Nutr 78, 65–71.
Finnegan YE, Howarth D, Minihane AM, Kew S, Miller GJ, Calder FC et al. (2003). Plant and marine derived (n-3) polyunsaturated fatty acids do not affect blood coagulation and fibrinolytic factors in moderately hyperlipidemic humans. J Nutr 133, 2210–2213.
Finnegan YE, Minihane AM, Leigh-Firbank EC, Kew S, Meijer GW, Muggli R et al. (2003). Plant- and marine-derived n-3 polyunsaturated fatty acids have differential effects on fasting and postprandial blood lipid concentrations and on the susceptibility of LDL to oxidative modification in moderately hyperlipidemic subjects. Am J Clin Nutr 77, 783–795.
Francois CA, Connor SL, Bolewicz LC, Connor WE (2003). Supplementing lactating women with flaxseed oil does not increase docosahexaenoic acid in their milk. Am J Clin Nutr 277, 226–233.
Freese R, Mutanen M (1997). Linolenic acid and marine long-chain n-3 fatty acids differ only slightly in their effects on hemostatic factors in healthy subjects. Am J Clin Nutr 66, 591–598.
Goyens PL, Mensink RP (2006). Effects of alpha-linolenic acid versus those of EPA/DHA on cardiovascular risk markers in healthy elderly subjects. Eur J Clin Nutr 60, 978–984.
Harper CR, Edwards MJ, DeFilipis AP, Jacobson TA (2006a). Flaxseed oil increases the plasma concentrations of cardioprotective (n-3) fatty acids in humans. J Nutr 136, 83–87.
Harper CR, Edwards MC, Jacobson TA (2006b). Flaxseed oil supplementation does not affect lipoprotein concentration or particle size in human subjects. J Nutr 136, 2844–2848.
Kaul N, Kreml R, Austria JA, Landry MN, Edel AL, Dibrov E et al. (2008). A comparison of fish oil, flaxseed oil and hempseed oil supplementation on selected parameters of cardiovascular health in healthy volunteers. J Am Coll Nutr 27, 51–58.
Kelly DS, Nelson JG, Love JE, Branch LB, Taylor PC, Schmidt PC et al. (1993). Dietary alpha-linolenic acid alters tissue fatty acid composition, but not blood lipids, lipoproteins or coagulation status in humans. Lipids 28, 533–537.
Lemay A, Dodin S, Kadri N, Jacques H, Forest JC (2002). Flaxseed dietary supplement versus hormone replacement therapy in hypercholesterolemic menopausal women. Obstet Gynecol 100, 495–504.
Metcalf RG, James MJ, Mantzioris E, Cleland LG (2003). A practical approach to increasing intakes of n-3 polyunsaturated fatty acids: use of novel foods enriched with n-3 fats. Eur J Clin Nutr 57, 1605–1612.
Nestel PJ, Pomeroy SE, Sasahara T, Yamashita T, Liang YL, Dart AM et al. (1997). Arterial compliance in obese subjects is improved with dietary plant n-3 fatty acid from flaxseed oil despite increased LDL oxidizability. Arterioscler Thromb Vasc Biol 17, 1163–1170.
Rallidis LS, Paschos G, Liakos GK, Velissaridou AH, Anastasiadis G, Zampelas A (2003). Dietary alpha-linolenic acid decreases C-reactive protein, serum amyloid A and interleukin-6 in dyslipidaemic patients. Atherosclerosis 167, 237–242.
Singer P, Berger I, Wirth M, Godicke W, Jaeger W, Voigt S (1986). Slow desaturation and elongation of linoleic and alpha-linolenic acids as a rationale of eicosapentaenoic acid-rich diet to lower blood pressure and serum lipids in normal, hypertensive and hyperlipemic subjects. Prostaglandins Leukot Med 24, 173–193.
Wallace FA, Miles EA, Calder PC (2003). Comparison of the effects of linseed oil and different doses of fish oil on mononuclear cell function in healthy human subjects. Br J Nutr 89, 679–689.
Zhao G, Etherton TD, Martin KR, West SG, Gillies PJ, Kris-Etherton PM (2004). Dietary alpha- linolenic acid reduces inflammatory and lipid cardiovascular risk factors in hypercholesterolemic men and women. J Nutr 134, 2991–2997.
Acknowledgements
This study was supported by Flax2015, CIHR and the St Boniface Hospital and Research Foundation. Drs D Rodriguez-Leyva, MN Chahine and Ms CMC Dupasquier were a Visiting Scientist, Postdoctoral Fellow, and Trainee, respectively, of the Heart and Stroke Foundation of Canada.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Patenaude, A., Rodriguez-Leyva, D., Edel, A. et al. Bioavailability of α-linolenic acid from flaxseed diets as a function of the age of the subject. Eur J Clin Nutr 63, 1123–1129 (2009). https://doi.org/10.1038/ejcn.2009.41
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ejcn.2009.41
Keywords
This article is cited by
-
Effect of essential fatty acid blend on circadian variations of ambulatory blood pressure in patients with essential hypertension and coronary artery disease risk markers
Journal of Human Hypertension (2022)
-
Consumption of echium oil increases EPA and DPA in blood fractions more efficiently compared to linseed oil in humans
Lipids in Health and Disease (2016)
-
The effect of flaxseed dose on circulating concentrations of alpha-linolenic acid and secoisolariciresinol diglucoside derived enterolignans in young, healthy adults
European Journal of Nutrition (2016)
-
A review of the effect of omega-3 polyunsaturated fatty acids on blood triacylglycerol levels in normolipidemic and borderline hyperlipidemic individuals
Lipids in Health and Disease (2015)
-
Development of botanical and fish oil standard reference materials for fatty acids
Analytical and Bioanalytical Chemistry (2013)