The Korea Drug Development Fund (KDDF) is the largest public funder of drug research and development (R&D) in Korea. KDDF invests more than $150 million per year in promising projects across almost all therapeutic areas and modalities. The fund was established to enhance the competitiveness of the Korean biopharma sector, encourage innovation in drug development and take new drugs to the global market, ultimately contributing to global health.
KDDF provides R&D grants to approximately 100 new companies every year to help them translate early-stage drug discovery to clinical development. It also provides business development support, fosters international collaborations, and facilitates the transfer of technologies and knowledge to increase the success rate of the drug development process.
Since 2021, KDDF has invested in 345 projects, of which 172 have focused on cancer. Most projects in KDDF’s oncology pipeline are in the discovery stage and span a range of drug modalities, including molecules, antibodies, and cell/gene therapies (Fig. 1).
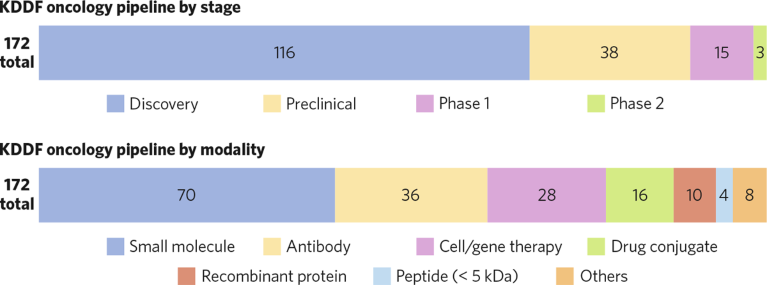
Fig. 1 | KDDF’s oncology pipeline. Candidates are shown by stage (top) and modality (bottom), January 2024.
KDDF recognizes that novel targets and modalities are of paramount importance in global drug development. Out of the 172 ongoing oncology projects, 76 feature either novel targets or innovative drug modalities.
KDDF actively seeks opportunities for co-development and other partnerships with global pharma companies on behalf of Korean biopharma companies to maximize the potential of their assets. Presented below are three companies that are translating cutting-edge science into novel cancer therapies.
PimedBio: blocking cancer stemness with PMB212
PimedBio, established in 2017, has developed PMB212, a first-in-class cancer-stemness blocking agent. PMB212 noncompetitively inhibits the enzyme PIN1, which upregulates more than 50 oncogenic proteins and downregulates more than 20 tumor suppressors. PIN1 is overexpressed in cancer cells of most types, particularly those with high self-renewal capacity or stemness that drive metastasis and therapeutic resistance. Despite participating in many pathways, PIN1 knockout mice grow normally. PMB212 blocks most of the signaling pathways related to cancer stemness in cells, including those mediated by Wnt, Notch, Hedgehog, Hippo, and STAT3. Moreover, PMB212 prevents epithelial to mesenchymal transition (EMT) and the expression of cyclinD1 and ABC transporters, which are implicated in metastatic activity and cancer progression.
PMB212’s ability to target multiple signaling pathways contributes to overcome the heterogeneity of cancer stemness and distinguishes PMB212 from other cancer stem cell-targeted candidates that selectively inhibit only one pathway. In animal models of breast, ovarian, and pancreatic cancer, PMB212 inhibited tumor growth and blocked peritoneal and vascular metastasis. Good laboratory practices (GLP) toxicity studies reported no observed adverse effects.
The multiple actions and good safety profile of PMB212 make it a promising agent for controlling cancer stemness, a major cause of cancer treatment failure. PMB212 may be beneficial in treating cold tumors that go undetected by the immune system and cancers where cancer stemness drives disease progression. PimedBio recently submitted an investigational new drug (IND) application for PMB212.
AbTis: overcoming limitations of antibody–drug conjugate linkers
AbTis, founded in 2016 and acquired by Dong-A ST in 2023, is a biotechnology company specialized in site-selective antibody conjugation. Its antibody–drug conjugate (ADC) linker technology, AbClick, enables cost-effective ADC production as it is applicable to ‘off-the-shelf’ antibodies and does not alter the inherent characteristics of the antibody.
AbTis is using AbClick to develop its own ADC pipeline focusing on solid tumors, as well as collaborating with global partners. The company’s lead candidate, AT-211, is an ADC against CLDN18.2, a transmembrane protein that is frequently overexpressed on the surface of gastric adenocarcinoma cells during the process of malignant transformation.
In CLDN18.2-positive cancer cell lines and a cancer cell-derived xenograft rodent model, AT-211 was shown to selectively bind CLDN18.2 and inhibit cell proliferation. AT-211 also showed a good pharmacokinetic profile in rats. In non-GLP studies, AT-211 demonstrated an excellent safety profile with a high therapeutic index.
AT-211 is expected to receive United States Food and Drug Administration (FDA) IND approval by the end of 2024. A protocol for a phase 1 clinical trial is currently being developed to evaluate the efficacy of AT-211 in gastric cancer patients in 2025.
CellBion: pioneering radioligand therapies
CellBion was established in 2010 and has rapidly positioned itself as a leader in radioligand therapy. CellBion’s flagship radioligand therapy Lu-177-DGUL selectively binds to the prostate-specific membrane antigen (PSMA), enabling targeted delivery of the therapeutic radioisotope to prostate cancer cells. Currently in phase 2 clinical trials (NCT05547061), Lu-177-DGUL has been granted orphan drug designation in Korea and has been designated for fast-track approval by the Korean Ministry of Food and Drug Safety.
The company’s pipeline also includes radioligands for imaging macrophages and is making advances in the field of antibody–radionuclide conjugates, which combine the high selectivity of antibodies with rapid excretion from the body, thus enhancing both safety and efficacy.
CellBion is also establishing a center of excellence in Korea for radioligand therapy. Recognizing the critical importance of supply chain infrastructure in this field, CellBion is in collaboration with global partners and utilizing Korea’s nuclear reactors to manage the production and supply of radioisotopes to help ensure the supply of radioligand therapies in Asia and beyond.


