Glioblastoma (GBM) is a fast growing and aggressive brain cancer that has a high mortality rate. Median survival is 16 months, with only around 5% of patients surviving more than five years. Surgery and radiation therapy are important for the treatment of GBM, and chemotherapy is helpful in some patients. However, tumor recurrence often results from inability to control microscopic residual disease.
Neuro-oncology researcher Michael Ciesielski and neurosurgeon Robert Fenstermaker began their work on the SurVaxM vaccine at Roswell Park Comprehensive Cancer Center. Ciesielski and Fenstermaker, along with their team, discovered that by targeting the anti-apoptosis protein survivin they could use a patient’s own immune system to attack residual tumor tissue left behind after surgery.
“Based on extensive laboratory studies, we realized that survivin-targeted immunotherapy had great potential to help patients with cancer; however, the best way to realize that potential was to form a company. Out of this, MimiVax was born to develop our findings and initiate clinical trials,” said Fenstermaker, who is a co-founder and CMO of the privately held, clinical-stage biotech based in Buffalo, NY.
Triggering an immune response against tumor-specific antigens
In healthy tissues, survivin is usually only expressed during fetal development. However, it is also found in virtually all GBMs and many other cancers. Thus, survivin may be broadly useful as an immunotherapy target. Survivin is known to be immunosuppressive, facilitating a tumor’s ability to evade the body’s immune response. Increased levels of survivin also correlate with poor response to chemotherapy and increased risk of cancer recurrence.
MimiVax’s lead vaccine candidate, SurVaxM, combines a survivin peptide mimic modified to enhance immune responsiveness by acting as a bioconjugate immunostimulant (Fig. 1).
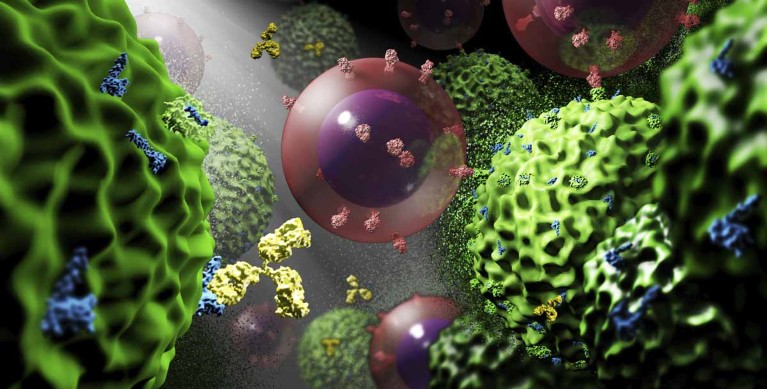
Fig. 1 | Artist’s interpretation of SurVaxM response at the cellular level. Residual glioblastoma cells (green) are attacked by vaccine-induced humoral and cellular responses. Antibodies (gold) directly attach to surface-bound survivin (blue); T cells (pink) target glioblastoma cells through engagement of their T cell receptors with tumors’ HLA-presented survivin peptide. Credit: S. Figel
In clinical trials, SurVaxM is administered after surgery and radiotherapy, when the tumor burden is low and the immune system is known to be more responsive. Administered subcutaneously once every two weeks for four doses with a maintenance dose every eight weeks thereafter, SurVaxM triggers antibody, T cell and cytokine responses designed to target survivin in and on cancer cells. SurVaxM was granted an orphan drug designation and was recently awarded a fast track designation from the US Food and Drug Administration (FDA) for newly diagnosed GBMs.
MimiVax has completed a phase 2a trial in newly diagnosed GBMs using SurVaxM as an add-on to standard therapy. The median overall survival reported in the previous phase 2a study was 28.4 months from diagnosis. The immune response to SurVaxM shows several characteristics that may ultimately provide useful response biomarkers.
A larger follow-up prospective randomized phase 2b trial, titled SurVaxM Plus Adjuvant Temozolomide for Newly Diagnosed Glioblastoma (SURVIVE), began in 2022 and is now ongoing at 11 cancer centers in the USA.
“There aren’t many therapeutic options for newly diagnosed GBM, and so the SURVIVE trial has been one of the fastest enrolling studies for this diagnosis, with two-thirds of the patients recruited in just six months,” said Ciesielski, MimiVax’s CEO.
Additional phase 1 studies of SurVaxM in recurrent GBM (RESURGe), multiple myeloma and neuroendocrine tumors are currently underway. A new phase 1 study of SurVaxM in pediatric relapsed/progressive medulloblastoma, high-grade glioma, ependymoma and newly diagnosed diffuse intrinsic pontine glioma is now recruiting in the USA and Canada, under the auspices of the Pediatric Brain Tumor Consortium.
“We believe that the potential of survivin-targeted therapy in cancer is highly significant,” said Fenstermaker.
Pipeline approach to anti-survivin immunotherapy
Retaining its focus on survivin, MimiVax is developing additional immunotherapeutic approaches in preclinical studies. These include a survivin monoclonal antibody platform with potential to target survivin-expressing cancers as well as autoimmune diseases such as myasthenia gravis. Also, a survivin-targeting chimeric antigen receptor (CAR)-T cell approach to survivin-expressing cancers isunder development.
Support
MimiVax’s goal is to make its therapies available to patients and healthcare professionals through FDA-regulated avenues by way of rigorously structured and monitored clinical trials. In 2019, MimiVax signed an exclusive licensing agreement with Fosun Pharma for the development and distribution of SurVaxM for GBM patients in China once it has received regulatory approval. MimiVax plans to expand its reach through new partnership and licensing opportunities with large biopharma companies.


