Algen Biotechnologies fuses cutting-edge gene editing clustered regularly interspaced short palindromic repeat (CRISPR) technology with artificial intelligence (AI) to reverse engineer and unravel the complex biology of cancer and other diseases to find novel, high-confidence therapeutic targets. Through deciphering the causal RNA signaling pathways that drive disease progression, Algen’s human-centric functional genomics platforms generate detailed maps to reveal the landscape of single-cell molecular phenotypes and help illuminate routes to novel therapeutics. Algen has a pipeline of six development programs—three in oncology, and three in immunology and metabolic disorders—with a lead anticancer agent being prepared for clinical trials.
Algen spun out of the laboratory of Jennifer Doudna, co-winner of the 2020 Nobel Prize in Chemistry for her pioneering work on CRISPR. The company has developed two core proprietary platform technologies to create a novel approach to target discovery and therapeutic development: AlgenCRISPR, a highly robust and tune-able gene-modulation system; and AlgenBrain, whose deep-learning models process huge quantities of precise gene-modulation data to guide target and compound screening in disease-driving cell types across multiple indications. Together, these platforms deconvolute complex biological pathways to identify high-confidence therapeutic targets.
AlgenBrain and AlgenCRISPR
AlgenBrain draws on billions of curated, fit-for-purpose, real-time dynamic changes in single-cell gene-expression data in disease-relevant cell types, from healthy to pathogenic and all intermediate stages drawn from human cohorts (Fig. 1). AlgenBrain’s proprietary deep learning uses these data to create powerful multidimensional representations of the molecular drivers of disease, providing intelligent insights in pathogenesis and the molecular basis of crucial cell-fate decisions to identify causal drivers of disease.
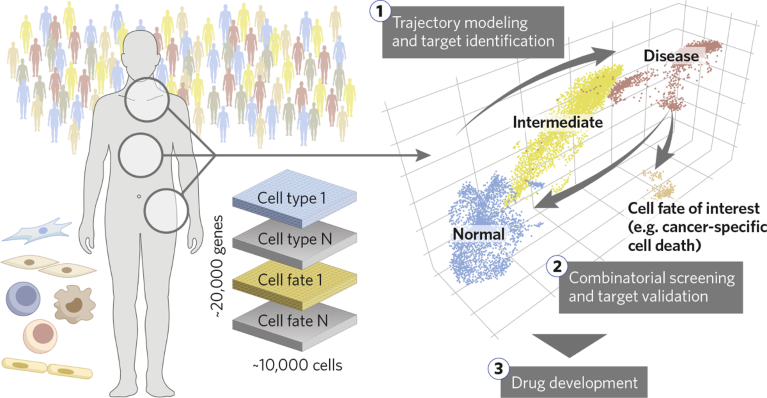
Fig. 1 | The Algen platform. Starting with human-centric, disease-relevant single-cell gene-expression data, AlgenBrain defines disease trajectories and therapeutic metrics, which narrows focus on to high-confidence targets that are priority ranked using AlgenCRISPR. Customized cell fates (for example, cancer-specific cell death) can be built into the latent space to understand CRISPR impact towards project-relevant phenotypes.
Gene targets in these pathways are then functionally explored using AlgenCRISPR for best-in-class modulation of gene expression, rather than the gene knockout typical of other CRISPR approaches that can induce DNA-damage responses that negatively impact screening outcomes and reproducibility. AlgenCRISPR, which has been engineered to lack endonuclease activity, does not create double-strand DNA breaks, and contains further modifications to the scaffold of single guide RNA (sgRNA) and an AI-powered predictive model for designing sgRNAs with optimal binding to desired genomic sites. AlgenCRISPR can be used to either up- or down-regulate gene expression with ease, and in head-to-head comparisons far outperforms other CRISPR-based gene-expression-modulation approaches, delivering up to 99% inhibition or a 2,000-fold increase in expression levels of target genes.
The effects of AlgenCRISPR gene modulation are then quantified at the single-cell level in hundreds of thousands of cells simultaneously, creating data that AlgenBrain uses to assign therapeutic metrics to each gene modulation—whether cells move away from a disease state towards an intermediate or healthy state or towards toxicity—as well as the magnitude of this shift. In other contexts, such as oncology, AlgenBrain identifies cells moving towards fates such as cancer-specific cell death or towards greater drug sensitivity and lower drug resistance.
Based on these functional outcomes and therapeutic metrics, AlgenBrain priority ranks targets for further investigation. In one program, AlgenBrain not only identified PARP1—a validated target of a recently approved anticancer agent now used in the clinic for BRCA-mutated metastatic pancreatic adenocarcinoma—as a high-confidence target, but also found nine more with even better ranked prospects. These targets have fed into Algen’s oncology programs focused on ‘tough to target’ solid tumors driven by well-known oncogenic pathways—MYC, RAS and MYCN—but targeting novel RNA components of these pathways.
Therapeutic indications
To date, AlgenCRISPR and AlgenBrain, which are disease-agnostic with applicability across indications, have fueled a development pipeline comprising three internal oncology programs, and three target discovery partnerships (two in immunology, the third in cardiovascular/metabolic disease). Beyond target identification, AlgenBrain is also ideally suited to priority ranking therapeutic candidates—small molecule, biologic or any other modality—by analyzing the genome-wide effects of a candidate intervention. By capturing these in the human-centric context of disease progression with therapeutic metrics, AlgenBrain provides a powerful tool for lead candidate identification and development.
Algen is committed to building a robust pipeline of internal programs and proprietary novel therapeutics, but also welcomes talks with pharma partners who wish to work with Algen to capitalize on the unique insights into disease and therapeutic potential afforded by AlgenCRISPR and AlgenBrain.


