For targeted cancer therapies to work, they must be delivered to the right patients: those with related tumor-associated biological changes. This requires identifying biomarkers that capture the relevant biology and can reliably guide treatment. Genialis is dedicated to developing next-generation biomarkers to realize the full potential of targeted therapies. It believes in the unique quality of RNA to deliver the right balance of information density and clinical utility to lead this biomarker revolution.
Despite more than a million biomarker publications in the literature, only about 30 have been approved by the US Food and Drug Administration (FDA) for use in companion diagnostic devices. New kinds of targeted therapies (such as immune-checkpoint inhibitors) convey extraordinary benefit to some patients but show little or no efficacy in others. There are just a few biomarkers available to inform treatment decisions, and these are limited in predicting how a patient will respond to a given drug. Further, most promising investigational drugs never reach patients at all, with a 97% oncology clinical-trial failure rate. Success is 5 to 12 times more likely, however, in biomarker-based clinical studies1.
Introducing ResponderID
For traditional biomarkers, the output is typically binary—such as the presence or absence of a given mutation that causes a proteomic alteration. Genialis’ approach is different and embraces the complexity of the underlying biology of cancer.
ResponderID, Genialis’ biomarker-discovery framework, leverages biomedical data and machine learning (ML) to help biopharma companies to develop more-effective drugs with a higher probability of clinical success, and diagnostics companies to deploy tests leading to better treatment decisions.
The ResponderID framework includes proprietary technologies (such as bioinformatics software and an ML sandbox), harmonized data assets (such as ML-ready omics and clinical metadata), and validated algorithms (such as published signatures and standard-of-care biomarkers).
ResponderID draws on multi-modal datasets, especially RNA sequencing (RNA-seq) data, and employs ML/artificial intelligence (AI) to analyze changes in tens or hundreds of genes relevant to disease pathology. The proprietary algorithms built into ResponderID then identify patterns of RNA transcription that characterize the underlying biology of disease states and the patient’s likely response to therapeutics (Fig. 1). By probing the interactions of the underlying disease biologies, ResponderID biomarkers reflect complex disease phenotypes.
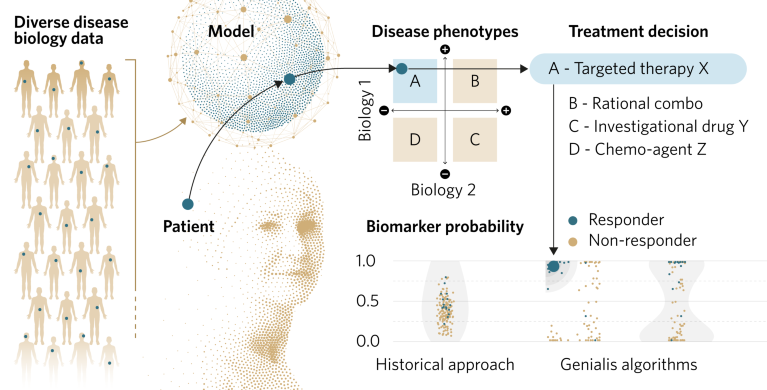
Fig. 1 | ResponderID. The people-first machine-learning platform for biomarker discovery.
In a notable application of this approach, Genialis worked with OncXerna Therapeutics to create the Xerna tumor microenvironment (TME) panel, a unique diagnostic tool for informing treatment decisions in cancer. The Xerna TME panel takes RNA-seq data as the input and employs an artificial neural network comprising about 100 genes to classify patient samples into one of four phenotypes based on the immune and angiogenic biologies of the TME.
A key attribute of the ResponderID approach is that each phenotype class predicted by the model has an associated therapeutic hypothesis (Fig. 1). For example, in the case of the Xerna TME panel, the immune and angiogenic biological axes define a quadrant of four TME subtypes: ‘Angiogenic’ (high angiogenic and low immune scores); ‘Immune suppressed’ (high angiogenic and high immune scores); ‘Immune active’ (low angiogenic and high immune scores); and ‘Immune desert’ (low angiogenic and low immune scores). Each TME subtype is predicted to respond differently to drugs depending on their mechanism of action (MoA). TMEs with a predominantly angiogenic profile, for example, would be expected to respond better to drugs based on an anti-angiogenic MoA than an immune-checkpoint inhibitor.
Collaborations and clinical trials
To date, ResponderID has been used to test the Xerna TME panel on real-world and clinical trial data from more than 15,000 patient samples representing 11 solid tumor types. Genialis, OncXerna, and other collaborators, such as Exact Sciences, Moffitt Cancer Center, and Royal Marsden, have presented data demonstrating the Xerna TME panel’s potential utility across numerous solid tumor types, based on retrospective analysis of six drugs (three approved and three investigational) with different targets and MoAs, and five different expression platforms. The Xerna TME panel is now being developed as a companion diagnostic, as well as a laboratory-developed test (LDT) for clinical research and trial recruitment.
The Xerna use case demonstrates how Genialis’ biology-first approach yields biomarkers that have pan-cancer potential and are applicable to entire classes of drugs rather than just single agents, thus increasing their utility for diagnostic and drug development applications. Further, these biomarkers aim to deliver a complete picture, identifying not just which class of drug is most suitable for a given patient but also why that is, based on the patient’s tumor biology. This contrasts with other biomarkers that are either insufficiently predictive in guiding treatment decisions or only provide a yes/no recommendation, where the ‘no’s’ have few, if any, good options.
By providing deep insights into the biology of a patient’s disease, ResponderID biomarkers can ensure that clinical trials recruit patients most likely to respond well to the MoA of an investigational drug, increasing the likelihood of trial success. Genialis welcomes discussions with collaborators in both diagnostic and biopharma sectors about deploying biomarkers to expedite all stages of clinical development, support approval of emerging therapeutics, and reach patients across disease settings. Together we can ensure that every patient gets the best possible treatment today and in the future.


