Systemic radioactive medicines as therapies for cancer were first employed in the 1940s, when physicians utilized radioactive iodine for the treatment of thyroid cancer. However, given the lack of potency of early radiotherapies and the side effects owing to their lack of specificity, scientists and clinicians have worked to develop safer and more potent medicines by linking radioactive isotopes to targeting moieties, such as tumor-specific small molecules, peptides or antibodies1.
The advent of commercially available antibody-directed radiotherapeutics began at the turn of this century with the approval of Zevalin (ibritumomab tiuxetan; now marketed by Acrotech Biopharma) and Bexxar (tositumomab and iodine-131 [I-131] tositumomab; developed by GlaxoSmithKline) for the treatment of non-Hodgkin’s lymphoma in 2002 and 2003, respectively. Despite demonstrated efficacy, and enthusiasm from physicians, several key challenges—including the need for specialized facilities, equipment and expertise for production and handling—limited their commercial success. Sales of Zevalin and Bexxar remained low for over a decade post-launch, leading to multiple changes in ownership for Zevalin, and Bexxar’s removal from the market.
The second-generation radiopharmaceuticals Xofigo (radium-223 [Ra-223] dichloride; Bayer, approved in 2013), Lutathera (lutetium-177 [Lu-177] dotatate; Advanced Accelerator Applications, approved in 2018) and Pluvicto (lutetium-177 [Lu-177] vipivotide tetraxetan; Novartis, approved in 2022) have been commercially successful. Indeed, Pluvicto, which is approved for the treatment of metastatic castration-resistant prostate cancer, is already on track to become a blockbuster, with sales of more than $250 million reported by Novartis in the third fiscal quarter (Q3) of 2023. Improvements in efficacy over existing standards of care, evolving oncology practices to include radiation oncologists, and the use of radioisotopes with improved stability and safety have converged to reinvigorate the field.
Moreover, the use of radiopharmaceuticals increasingly extends beyond therapeutics and into the field of diagnostics. Diagnostic agents containing a radioactive component, which accumulate in specific organs, tissues or cells and enable the detection of abnormalities or functional changes, have long been used in the assessment of tumors, neurological disorders, inflammatory diseases and infections2. Recently, there has been a significant push in the development of theranostic radiopharmaceuticals, which combine both diagnostic and therapeutic properties, supporting a ‘see and treat’ approach. Initially, a diagnostic component is used to visualize and assess a disease or condition, and then the same or a complementary radiopharmaceutical is used for therapeutic purposes. The ability to combine diagnostics and therapy in a personalized manner holds great promise for improving patient outcomes, optimizing treatment strategies and advancing precision-medicine approaches.
While first-generation targeted radiopharmaceutical products struggled commercially, the advent of more effective, safer and easier-to-use agents with strong commercial potential has spurred a renewal of interest in the field, leading to a flurry of recent licensing partnerships, mergers and acquisitions (M&As) and venture capital (VC) financing. Given this industry interest, we assessed the transactional and investment landscape within the radiopharmaceuticals space over the past 5 years, spanning therapeutics, diagnostics and theranostics.
Radiopharmaceutical deals
Over the past 5 years, there have been 86 strategic deals in the radiopharmaceuticals space, with the majority being collaboration/co-development (n = 26) and licensing-based (n = 26) agreements. The transaction landscape was relatively stable from 2018 to 2021. However, in 2022, the number of deals struck nearly tripled compared to 2021 (n = 25 and n = 9, respectively; Fig. 1a). Notably, the greatest number of deals was struck for assets in the preclinical phase (n = 20), primarily driven by firms with existing nuclear-medicine capabilities and expertise (such as Fusion Pharmaceuticals and Novartis) seeking to expand their radiopharmaceutical pipeline. Many deals also occurred at phase 2 (n = 18); these were predominately licensing and acquisition-based deals of diagnostic radiopharmaceuticals to be used for identifying patients best suited for the partner companies’ therapeutic asset(s) (Fig. 1a).
Active consolidators in the space range from global biopharma companies (such as Novartis and Bayer) to dedicated nuclear-medicine firms (such as Fusion Pharmaceuticals and Curium Pharma), with deal activity spread across diagnostic, theranostic and therapeutic agents. Diagnostic radiopharmaceuticals have commanded a considerable portion of the total number of deals in this space (~41%), followed by theranostics (~32%) and therapeutics (~27%; Fig. 1b). While oncology has dominated the theranostics and therapeutics deal spaces (with 93% and 100% of the deals, respectively), the largest portions of the deals in the diagnostics space have been in neurology (54%) followed by oncology (31%).
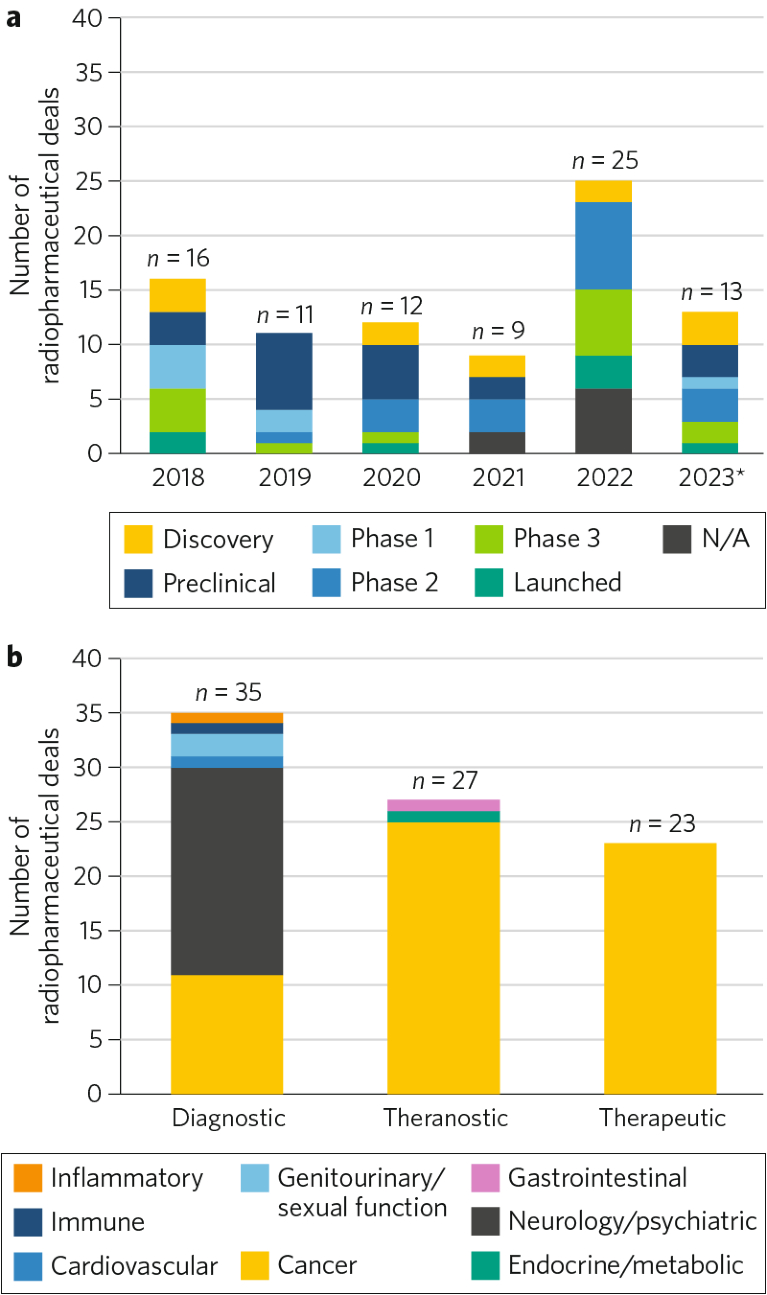
Fig. 1 | Biopharma-sponsored deal distribution in the radiopharmaceuticals space: 2018–2023*. a, Number of deals by phase, per year. b, Number of deals by indication, per modality. Data source: Cortellis. *Through 3 October 2023. N/A, not available.
Within the period analyzed, there were 17 deals with disclosed financial terms at the time of announcement (~20%). The average potential overall value for precommercial deals was $834 million ($284 million up-front, $550 million in milestones) across all stages of development and modalities (Fig. 2a). Compared to the average total deal value for diagnostic assets ($39 million; n = 4), the potential deal values for theranostic (n = 4) and therapeutic (n = 9) assets were considerably greater at $729 million and $1,026 million, respectively. The average up-front deal value for theranostic assets was ~2 times that of therapeutic assets, which may be attributed to the lower clinical and regulatory risks of the former.
When considering deal value by maturity of asset, clinical-stage assets commanded increasing value as the technology progressed through the clinic, though compared to early-stage clinical assets, discovery-stage deals achieved a higher total deal value (Fig. 2b). The majority (97%) of the value for deals struck in the discovery phase lay in the milestone payments, reflecting the high-risk, high-reward nature of these collaboration-based deals for the discovery, development and commercialization of radiopharmaceutical therapies.
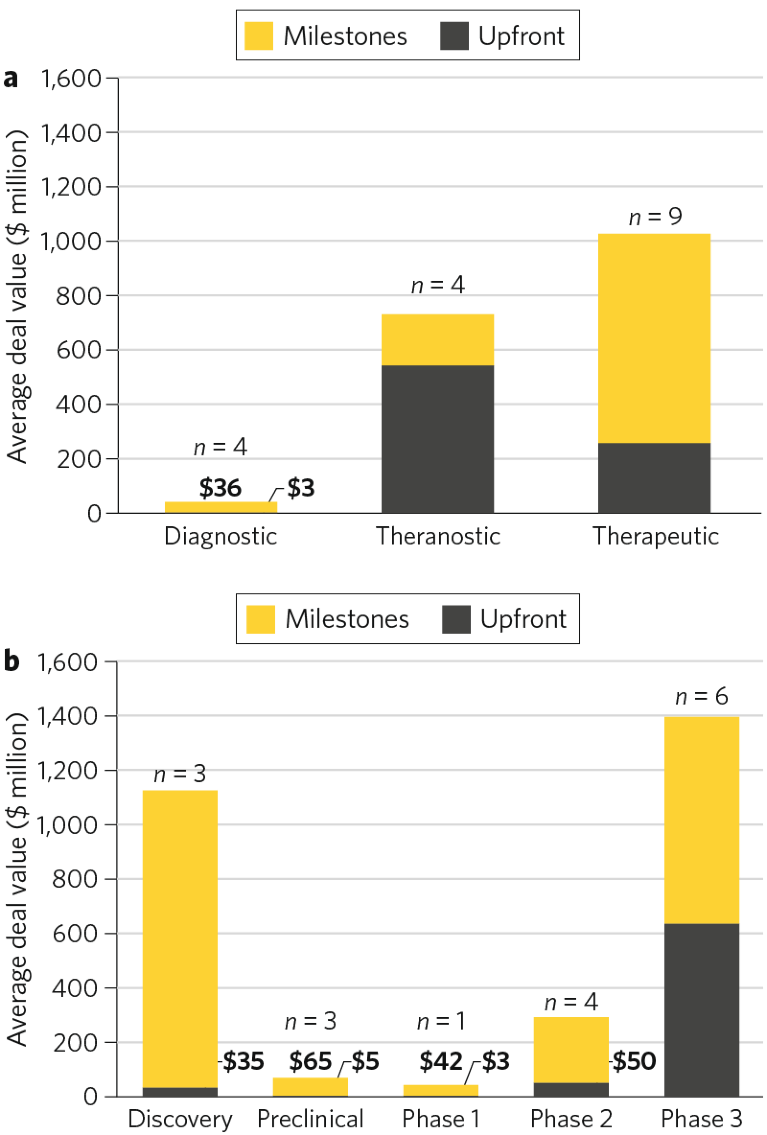
Fig. 2 | Biopharma-sponsored deal values in the radiopharmaceuticals space: 2018–2023*. a, Value of deals with disclosed terms, by modality. b, Value of deals with disclosed terms, by phase. Data source: Cortellis. *Through 3 October 2023.
Deals at the commercial stage have primarily been M&As, acquiring companies with recent US Food and Drug Administration (FDA) approvals. Notably, Novartis paid $3.9 billion upfront to acquire Advanced Accelerator Applications in 2018, close on the heels of the FDA approval of the neuroendocrine tumor (NET) diagnostic Netspot (gallium [ga-68] dotatate kit), and almost coincident with the FDA approval of the therapeutic Lutathera.
Company financings
Since 2018, the number of financings occurring in the radiopharmaceuticals space (n = 54) has been steadily increasing, with a predominant focus on cancer indications (n = 45; Fig. 3). Theranostics is the most active modality for financing (50%), followed by therapeutics (34%) and diagnostics (16%), with most of the financings occurring early, at series A (56%) and B (32%). As interest and clinical development have been rising, so too has the level of financing; the average financing across nine raises in the first three quarters of 2023 was $51 million, ~2 times greater than that in the whole of 2018, at $26 million across four raises (Fig. 3).
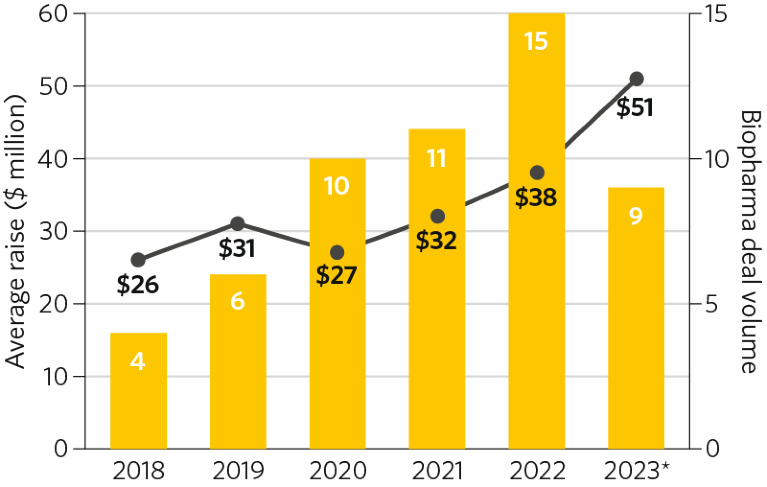
Fig. 3 | Venture-capital-backed private financings in the radiopharmaceuticals space: 2018–2023*. Financings are from series A to D. Data source: Pitchbook. *Through 3 October 2023.
Performance of public companies
While the enthusiasm surrounding radiopharmaceuticals has led to an increase in dealmaking, the performance of the five radiopharmaceutical companies that were listed from the start of 2023 on major US exchanges (the NYSE or Nasdaq)—Fusion Pharmaceuticals, Lantheus Medical Imaging, POINT Biopharma, Perspective Therapeutics and Actinium Pharmaceuticals—has been mixed. From the beginning of the year to 3 October 2023, their performance ranged from a rise in share price of 71% for POINT Biopharma to a fall of 43% for Actinium Pharmaceuticals. The share price of the median company in the group of five, Perspective Therapeutics, increased by 11%, considerably more than the Nasdaq Biotechnology Index, which returned −7% over the same timeframe.
One possible explanation for the positive performance of public radiopharmaceutical companies on average was the excitement surrounding the modalities in the private markets spilling over into the public market. Reinforcing this theory was the initial public offering (IPO) for RayzeBio, a radiopharmaceutical therapeutic company with a lead asset, actinium-225 (Ac-225) dotatate, in phase 3 development for the treatment of gastroenteropancreatic NETs. The company raised $358 million when it went public on the Nasdaq on 15 September 2023, giving it a valuation of almost $1 billion after its listing. This was the second largest biotech IPO of the year to date, behind Acelyrin’s $540 million raise, and was ~2.9 times the average healthcare IPO size in 2023.
By the end of 2023, two public radiopharmaceutical companies had entered into agreements to be acquired, as two more major pharma companies bought into the field, joining Novartis and Bayer. On 26 December 2023, RayzeBio entered into a definitive merger agreement with Bristol Myers Squibb to be acquired for $4.1 billion, reflecting a 134% premium on RayzeBio’s share price at the time. One day later, Eli Lilly announced that it had closed its acquisition of POINT Biopharma for $1.4 billion, which was an 87% premium on POINT’s closing share price a day before the acquisition was announced on 3 October 2023. The willingness of large pharma companies to pay high premiums for radiopharmaceutical companies further demonstrates the burgeoning interest in the field.
Looking ahead
With a significant amount of investment being poured into early-stage radiopharmaceutical companies, we expect pharma to continue their licensing and M&A activity as these novel technologies mature. Moreover, with the increasing emphasis on personalized medicine, theranostics are likely to continue to be of keen interest; indeed, theranostics commanded the greatest upfront value of the modalities considered in our analysis. The use of radiopharmaceuticals in areas beyond oncology is likely to bring additional companies and VCs to the table. In fact, neurology was the leader when it came to diagnostic deals owing to radiopharmaceuticals’ unique ability for visualizing the brain and other parts of the central nervous system. Overall, the radiopharmaceuticals space is charged with anticipation and high expectations as deals continue at pace.