Acute respiratory-distress syndrome (ARDS) affects around 10% of people in intensive-care units (ICUs) and occurs in all age groups from newborns to the elderly. It can develop following bacterial or viral infections or trauma. It has a high risk of long-term damage and death; mortality is 32–45% in patients with moderate-to-severe ARDS.
“In ARDS, the membranes become ‘leaky’—this means that patients have trouble oxygenating, lungs fill with fluid, and they effectively drown. There was an increased awareness of ARDS during the COVID-19 pandemic, but the area has quietened again. We feel that it’s important to retain the focus, as ARDS remains a key issue in ICUs for patients with viral infections, pneumonia and sepsis, and there will be further pandemics in the future,” said Brian Jahns, president and COO of Vasomune.
Private Canadian biotechnology company Vasomune Therapeutics was created in 2014 as a spinout from Sunnybrook Research Institute to develop AV-001 (also referred to as vasculotide) as a potential therapeutic to reduce pulmonary edema in ARDS and improve survival. Vasomune Therapeutics has global patent protection until 2038 spanning composition and methods of use.
Creating a therapeutic
At the root of ARDS is the host vascular response (HVR), an inflammatory response to injury, which results in vascular leakage and edema in organs including the lungs. This can lead to multi-organ failure and death. Daniel Dumont of Sunnybrook Research Institute discovered and characterized Tie2, a tyrosine-protein kinase receptor expressed on the surface of endothelial cells in the vasculature. Tie2 and its endogenous ligand angiopoietin-1 play important roles in regulating barrier defense, the HVR to injury, and maintaining vascular homeostasis.
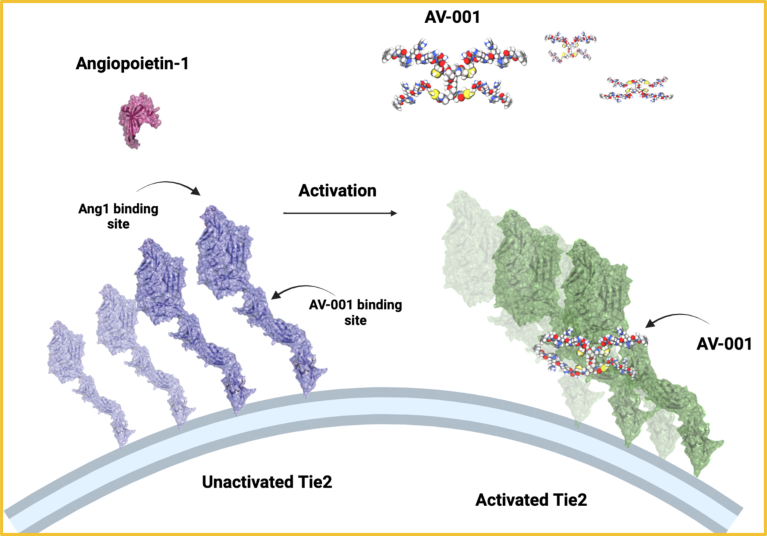
Fig. 1 | AV-001 can be used across therapeutic areas affected by vascular destabilization. AV-001 uniquely activates Tie2 in order to promote endothelial stabilization.
AV-001 is a fully synthetic angiopoietin-1 mimetic invented by Dumont and his colleague Paul Van Slyke (Fig. 1). With the goal of taking the potential therapeutic from basic research and discovery through preclinical trials to the patient’s bedside, Vasomune Therapeutics was created through a collaboration between Sunnybrook Health Sciences Centre and Toronto Innovation Acceleration Partners (TIAP).
“The aim of AV-001, our first-in-class injectable Tie2 selective-receptor agonist, is to stabilize and normalize vascular dysfunction, blocking vascular leak,” said Harold Kim, VP of Research and Scientific Affairs at Vasomune Therapeutics. “Our initial indication is ARDS resulting from infection, however AV-001 has the potential to be used in other diseases where vascular leak forms an underlying pathophysiology.”
In 2018, Vasomune signed a collaboration deal with Japan-based pharmaceutical company AnGes, Inc. The co-development deal was set up to move AV-001 into clinical trials, with the companies sharing development expertise and expenses.
“Our collaboration with AnGes, Inc. has been very important. Not only has it provided financial support, it has also given us access to business expertise and clinical development support,” said Jahns.
As a threat-agnostic and host-directed therapeutic, AV-001 has potential to address ARDS from known or unknown health-security threats, such as global pandemics and other emerging infectious-disease threats.
“Funding from the US Department of Defense (DoD) and the National Research Council of Canada Industrial Research Assistance Program (NRC IRAP) shows that governments and institutions recognize the impact of global pandemics and emerging infectious-disease threats on national health security, and the importance of countermeasures such as AV-001,” said Kim. “The biopharma industry must not forget that we have recently survived a global infectious-disease pandemic, and we are likely to experience another. Drugs for the sequelae of infectious diseases need to remain a priority moving forward.”
Moving towards the market
Clinical trials of AV-001 began in December 2020, with preliminary results showing that it was safe and well tolerated.
Vasomune’s IND-enabling work was supported, in large part, by the US DoD. The successful phase 1 study was supported by NRC IRAP, with phase 2a trials being supported by the US DoD in patients with pathogen-induced ARDS. Vasomune expects completion of the phase 2a trial by the end of 2024. A phase 3 trial is planned, to support a new drug application (NDA) in pathogen-induced ARDS.
Vascular endothelial dysfunction is also at the core of many other conditions, and so AV-001 may also have potential in acute kidney injury, acute lung injury, cardiopulmonary bypass surgery, stroke, endotoxemia, sepsis, hemorrhagic shock, hemorrhagic fever, vascular dementia, dermal injury and burns.
“We’ve effectively challenged the scientific community’s long-held belief that Tie2 is undruggable,” said Jahns. “Based on our strong preclinical data, and the momentum created with our clinical program, Vasomune shareholders are justified in their high expectations for AV-001.


